Question: A monatomic gas follows the process 1 2 3 shown in Figure EX19.32. How much heat is needed for (a) Process 1 2 (b) Process
A monatomic gas follows the process 1 †’ 2 †’ 3 shown in Figure EX19.32. How much heat is needed for
(a) Process 1 †’ 2
(b) Process 2 †’ 3?
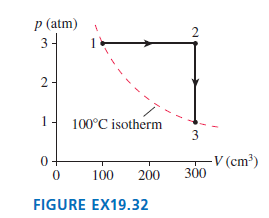
p (atm) 3- 2- 100C isotherm 3 -V (cm) 300 100 200 FIGURE EX19.32
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Model The gas is assumed to be an ideal gas that is subje... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778b5b60f_690305.pdf
180 KBs PDF File
1442_6054778b5b60f_690305.docx
120 KBs Word File


