Question: Sketch the n = 1 and n = 7 wave functions for the potential energy shown in FIGURE EX40.15. U(x) E, E FIGURE EX40.15
Sketch the n = 1 and n = 7 wave functions for the potential energy shown in FIGURE EX40.15.
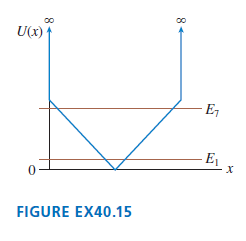
U(x) E, E FIGURE EX40.15
Step by Step Solution
3.36 Rating (165 Votes )
There are 3 Steps involved in it
Visualize The steps of ... View full answer

Get step-by-step solutions from verified subject matter experts


