You are given the equation(s) used to solve a problem. For each of these, you are to
Question:
a. Write a realistic problem for which this is the correct equation(s).
b. Draw a pV diagram.
c. Finish the solution of the problem.
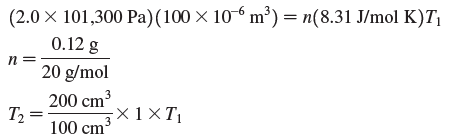
Transcribed Image Text:
(2.0 × 101,300 Pa)(100 × 10-6 m³) = n(8.31 J/mol K)T| 0.12 g 20 g/mol п 200 cm3 ×1 ×T T2 = 100 cm 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Solve a 012 g of neon gas at 20 atm and 100 cm 3 exp...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
You are given the equation(s) used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation(s). b. Draw a pV diagram. c. Finish the...
-
You are given the equation(s) used to solve a problem. For each of these, you are to a. Write a realistic problem for which this is the correct equation(s). b. Draw a pV diagram. c. Finish the...
-
You are given the equation(s) used to solve a problem. For each of these, a. Write a realistic problem for which this is the correct equation(s). b. Finish the solution of the problem. (9.0 10 Nm/C)...
-
5. Did Danone follow the advice regarding JVs in China mentioned in the list just above? Which aspects did it follow and which did it not? Danone's Wrangle with Wahaha In 1996, In 1996, Danone Group...
-
Determine the percentage of vesting for the following employees. See Exhibits 19.1 and 19.2. a. Glenda has five years of service completed as of September 23, 2016, her employment anniversary date....
-
Read the narrative for "Modart." below and Create a flowchart. Document the Request system."Modart is an art gallery that earns its revenue as an intermediary between artists and customers. Artists...
-
Distinguish between the following interest rates for bonds payable: (a) yield rate (d) market rate (b) nominal rate (e) effective rate (c) stated rate
-
Puckett Co. has office furniture that cost $75,000 and that has been depreciated $50,000. Record the disposal under the following assumptions. (a) It was scrapped as having no value. (b) It was sold...
-
Markets for Goods and Services Firms Households Markets for Factors of Production D Figure 1.2 Refer to Figure 1.2. Which arrow represents the flow of factors of production
-
A buffer solution was made up of ammonia and water which produced ammonium ions and hydroxide ions. a. Write the equilibrium equation which represents this buffer and the simplified Ka expression b....
-
Starwood Hotels (Starwood) owns and operates many hotel properties under well-known brand names, including Sheraton, W, Westin, and St. Regis. Starwood focuses on the upper end of the lodging...
-
Altman's bankruptcy risk model utilizes the values of the variables at a particular point in time (balance sheet variables) or for a period of time (income statement values). An alternative would be...
-
RCMP, Inc. shares rose 10 percent in value last year while the inflation rate was 3.5 percent. What was the real return on the stock? If an investor sold the stock after one year and paid taxes on...
-
Classic Auto Parts sells new and used auto parts. Although a majority of its sales are cash sales, it makes a significant amount of credit sales. During 2012, its first year of operations, Classic...
-
The following information is available for Market Inc. and Supply Inc. at December 31, 2012: Required a. What is the accounts receivable turnover for each of the companies for 2012 ? b. What is the...
-
Buck Novak, the chief executive officer of Novak Corporation, has assembled his top advisers to evaluate an investment opportunity. The advisers expect the company to pay \($400,000\) cash at the...
-
Verify the log-likelihood in equation (16.4) for the Tobit model. In L = = In { 1-0 (x-di)} 1:y=di 122. + (y; - x) 02 (16.4) i:y;>di
-
Milo Company is considering the purchase of new equipment for its factory. It will cost \($250,000\) and have a \($50,000\) salvage value in five years. 1 he annual net income from the equipment is...
-
Solve the given problems. The cross section of a large circular conduit has seven smaller equal circular conduits within it. The conduits are tangent to each other as shown in Fig. 2.93. What...
-
Suppose the market is semistrong form efficient. Can you expect to earn excess returns if you make trades based on? a. Your brokers information about record earnings for a stock? b. Rumors about a...
-
Two nuclei have different mass numbers A 1 and A 2 . Are the two nuclei necessarily isotopes of the same element? Explain.
-
What is the name of the force that holds protons and neutrons together in the nucleus?
-
What aspects of the composition of a nucleus can cause it to be unstable?
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


