A solid sample weighing 0.237 6 g contained only malonic acid and aniline hydrochloride. It required 34.02
Question:
A solid sample weighing 0.237 6 g contained only malonic acid and aniline hydrochloride. It required 34.02 mL of 0.087 71 M NaOH to neutralize the sample. Find the weight percent of each component in the solid mixture. The reactions are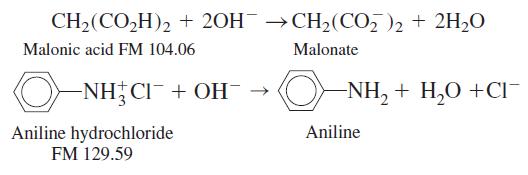
Transcribed Image Text:
CH2(CO,H)2 + 20H →CH2(CO, )2 + 2H,0 Malonic acid FM 104.06 Malonate -NH CI¯+ OH¯ → O- NH, + H,0 +CI¯ Aniline Aniline hydrochloride FM 129.59
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
3402 mL 08771 M NaOH 34020087711000 29810 3 mole of NaOH ...View the full answer

Answered By

TIRTHA MONDAL
I have completed my graduation and post graduation from Jadavpur University. I have also done Bachelor in Education (B.Ed) from the same university. I have experience in teaching JEE& NEET aspirants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solid mixture weighing 0.5485 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1M H2SO4, oxidized to Fe3+ with H2O2, and...
-
A mixture weighing 7.290 mg contained only cyclohexane, C6H12(FM 84.159), and oxirane, C2H4O (FM 44.053). When the mixture was analyzed by combustion analysis, 21.999 mg of CO2 (FM 44.010) were...
-
It takes 97.62 mL of 0.0546 M NaOH to titrate a 25.00 mL sample of H2SO4. What is the concentration of H2SO4? You will need to write the balanced chemical equation first.
-
The production of paper involves a pulping step to break down wood chips into cellulose and lignin. In the Kraft process, an aqueous, pulping-feed solution, known as white liquor, is used that...
-
Use the GSS 1OSSDS data file to study the relationship between the number of siblings a respondent has (SIBS) and his or her number of children (CHILDS). a. Construct a scatterplot of these two...
-
The sun emits energy in the form of electromagnetic waves at a rate of 3.9 x 1026 W. This energy is produced by nuclear reactions deep in the sun's interior. (a) Find the intensity of electromagnetic...
-
Independent random samples selected from two normal populations produced the sample means and standard deviations shown below: Sample 1 Sample 2 a. The test H,,: (p, - p2) = 0 against Ha: (p, - p2) f...
-
Theresa Thayer, a friend from college, asks you to form a partnership to import fragrances. Since graduating, Thayer has worked for the Spanish Embassy, developing important contacts among government...
-
The price (per $100 face value) of a 5% semi-annual pay bond with exactly 2-1/2 years to maturity and a yield to maturity of 5.75% is (a) 96.8191 (b) 86.2162 (c) 98.2765 (d) None of the above
-
Reece Financial Services Co., which specializes in appliance repair services, is owned and operated by Joni Reece. Reece Financial Services accounting clerk prepared the following unadjusted trial...
-
A solution of NaOH was standardized by gravimetric titration of a known quantity of the primary standard, potassium hydrogen phthalate: Potassium hydrogen phthalate C 8 H 5 O 4 K, FM 204.22 The NaOH...
-
A 50.0-mL sample of 0.080 0 M KSCN is titrated with 0.040 0 M Cu + . The solubility product of CuSCN is 4.8 10 -15 . At each of the following volumes of titrant, calculate pCu + , and construct a...
-
Suppose study of the human brain (science) leads to the development of a device (technology) that allows a user to both download information from another persons brain (mind-reading) and upload...
-
What is an incident in which a famous person wore or used a product (not as part of a paid endorsement or ad) and it caused a buying frenzy. Explain how the manufacturer or service provider reacted
-
What is a "heavyweight project team" and how does it differ from the traditional approach used for organizing development projects at Eli Lilly?This consists of two issues:First, an evaluation of the...
-
Consider the closed-loop system shown in Figure P11.6, where the transfer function of the process is that of a second-order system, i.e. k Ts +25TS +1 G,(s)= Y sp(s) E(s) U(s) Y(s) Ge(s) Gp(s) Figure...
-
1. Do you feel we have come along way with inventory in 10 years? 2. How did COVID affect the supply chain in your current hospital? Were any of the inventory systems/topics used, or relevant or...
-
Identify at least one way in which your writing skills have improved this semester and reflect on how you might use this skill in your career. You can include research, presentation, and report...
-
What is the amount of the tax liability for a married couple filing jointly with taxable income of $135,500? a. $29,810. b. $21,527. c. $18,200. d. $21,689.
-
Making use of the tables of atomic masses, find the velocity with which the products of the reaction B10 (n, ) Li7 come apart; the reaction proceeds via interaction of very slow neutrons with...
-
The voltage for the following cell is 0.490 V. Find Kb for the organic base RNH2. Pt(s) H2(1.00 bar) RNH2(aq, 0.10 M), RNH+3 Cl(aq, 0.050 M) ||' S.H.E.
-
The voltage of the cell shown here is - 0.246 V. The right half-cell contains the metal ion, M2+, whose standard reduction potential is - 0.266 V. Mc2+ + 2e - E° = - 0.266 V Calculate Kf for the...
-
The following cell was constructed to find the difference in Ksp between two naturally occurring forms of CaCO3(s), called calcite and aragonite.21 buffer(pH 7.00) CaCO3(s, aragonite) PbCO3(s) ...
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


