Calculate the pH of a 0.010 M solution of each amino acid in the form drawn here.
Question:
Calculate the pH of a 0.010 M solution of each amino acid in the form drawn here.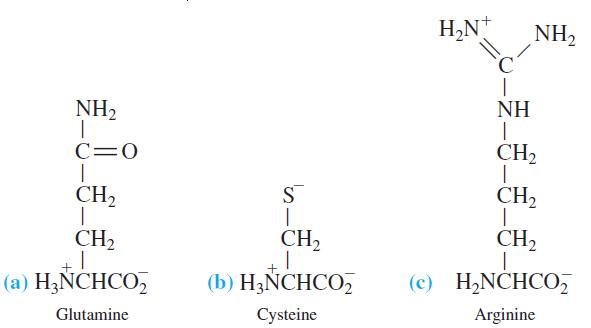
Transcribed Image Text:
H,N* NH2 NH2 NH C=0 CH2 CH2 S CH2 CH2 CH2 CH2 (a) H¿ÑCHCO, (b) H3ÑCHCO, (c) H,NCHCO, Glutamine Cysteine Arginine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Amina acid PH PH of each Solution 0010 M The three forms of glutamine is as follows L...View the full answer

Answered By

Manoj Kumar Mahariya
Teaching experience-
1.As a lecture in Akash Carrer institute (since 2 year)
2.As a assistant lecturer in National Institute of technology,Punjab
3.Also school lecture of pcbm subject in Ravindra manorial senior sec.school, Rajasthan
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of a solution prepared by (a) Dissolving 36.5 g of lactic acid in water and diluting to 500 mL. (b) Diluting 25.0 mL of the solution in (a) to 250 mL. (c) Diluting 10.0 mL of the...
-
Calculate the pH of a solution made by mixing 0.60 L of 0.10 M NH4Cl with 0.50 L of 0.10 M NaOH. Kb for NH3 is 1.8 10-5.
-
Calculate the pH of a solution made by mixing 7.52 mL of 4.9 10-2 M Ca(OH)2 with 22.5 mL of 0.11 M HCl.
-
The following information was taken from the annual manufacturing overhead cost budget of Granada Company. Variable manufacturing overhead costs .....$33,000 Fixed manufacturing overhead costs...
-
The GSS asked respondents to report their opinion on spanking as a method to discipline a child (SPANKING). Examine how respondents' attitudes toward spanking a child are associated with SEX, CLASS,...
-
A bus is parked along the side of a highway when it is passed by a truck traveling at a constant speed of 70 km/h. Two minutes later, the bus starts and accelerates until it reaches a speed of 100...
-
What is demand-based pricing? How can a foodservice operator find out what the market will bear?
-
Christie Realty loaned money and received the following notes during 2012. Requirements For each note, compute interest using a 360-day year. Explanations are not required. 1. Determine the due date...
-
Q1. Knowledge Transfer Associates is in the process of evaluating its new client services for the business systems consulting division. Server Planning, a new service, incurred $270,000 in...
-
Cherry Cotta makes custom ordered clay pots for residential gardens. Below is cost information regarding its latest job. a. Materials were purchased on account. $18,996 purchased b. A materials...
-
Interpreting spectral data. The graph shows the 1H-nuclear magnetic resonance chemical shift of the H 4 proton on pyridine as a function of pH. Chemical shift is related to the environment of a...
-
Phosphate at 0.01 M is one of the main buffers in blood plasma, whose pH is 7.45. Would phosphate be as useful if the plasma pH were 8.5?
-
Protein molecules are usually quite large and yet often water-soluble. They are soluble in water because they can fold themselves into spherical shapes in which the polar (hydrophilic) side chains...
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
Elizabeth determined that her tax liability was $3,492. Her employer withheld $3,942 from her paychecks during the year. Elizabeths tax return would show: a. A refund of $450. b. A refund of $3,942....
-
If a and b are positive numbers, find the maximum value of f ( x ) = x a (9 x ) b on the interval 0 x 9.
-
A 10.0-mL solution of 0.050 0 M AgNO 3 was titrated with 0.025 0 M NaBr in the cell S.C.E. 7 || titration solution | Ag(s) Find the cell voltage for 0.1 and 30.0 mL of titrant.
-
A solution containing 50.0 mL of 0.100 M EDTA buffered to pH 10.00 was titrated with 50.0 mL of 0.020 0 M Hg(ClO 4 ) 2 in the cell shown in Exercise 14-B: S.C.E. 7 titration solution | Hg(l) From the...
-
Consider the titration in Figure 15-2. (a) Write a balanced titration reaction. (b) Write two different half-reactions for the indicator electrode. (c) Write two different Nernst equations for the...
-
Metlock Limited has signed a lease agreement with Lantus Corp. to lease equipment with an expected lifespan of eight years, no estimated salvage value, and a cost to Lantus, the lessor of $170,000....
-
(International Finance) Computing a Currency changes = (e1 - e0 )/ e0 where e0 = old currency value e1 = new currency value (a) If the dinar devalues against the U.S. dollar by 45%, the U.S. dollar...
-
2. Fill in the time line for the Sawing Department. Use the time line to help you compute the number of equivalent units and the cost per equivalent unit in the Sawing Department for September Show...

Study smarter with the SolutionInn App


