Interpreting spectral data. The graph shows the 1H-nuclear magnetic resonance chemical shift of the H 4 proton
Question:
Interpreting spectral data. The graph shows the 1H-nuclear magnetic resonance chemical shift of the H4 proton on pyridine as a function of pH. Chemical shift is related to the environment of a proton in a molecule. If the environment changes, the chemical shift changes. Suggest an explanation for why the chemical shift changes between low pH and high pH. Estimate pKa for pyridinium ion (C5H5NH+).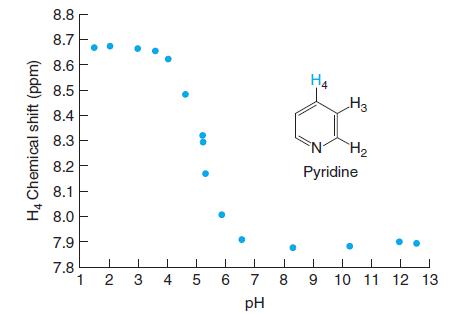
Transcribed Image Text:
8.8 8.7 8.6 H4 H3 8.5 8.4 8.3 H2 8.2 Pyridine 8.1 I 8.0 7.9 7.8 1 2 4 5 6 9 10 11 12 13 7 8 pH H4 Chemical shift (ppm) 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
As we all know that pyridine is a base Whenever it is in the environment of low pH ie in acidic envi...View the full answer

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Suggest an explanation for why liquid water is needed in an alkaline battery. (b) What is the advantage of using highly concentrated or solid reactants in a voltaic cell?
-
Suggest an explanation for the following observations: a. Although the hydrocarbon calicene has so far defied synthesis, but it has estimated that it would have a dipole moment as large as b. The...
-
(a) Suggest an explanation for the fact that 1-methylcyclopropene is some 42 kJ/mol (10 kcal/mol) less stable than methylenecyclopropane
-
How is it that a state, or any state, such as Oregon, can offer more provisions under FMLA (OFLA) than then the federal rule of FMLA does?
-
Illegal immigration in the United States is a complex matter, and people have diverse and conflicting ideas on how to best address it. The 2010 GSS contains several questions on this topic. For this...
-
A commuter train traveling at 64 km/h is 4.8 km from a station. The train then decelerates so that its speed is 32 km/h when it is 800 m from the station. Knowing that the train arrives at the...
-
What factors must be considered in setting the final prices of menu items?
-
Cannon Mountain Mining paid $462,300 for the right to extract mineral assets from a 400,000-ton deposit. In addition to the purchase price, Cannon also paid a $900 filing fee, a $1,800 license fee to...
-
Exercise 11-9A (Algo) Recording and reporting common and preferred stock transactions LO 11-4 Eastport Incorporated was organized on June 5. Year 1. It was authorized to issue 320,000 shares of $12...
-
Using the closed traverse below 1- Convert the traverse distances to a decimal of an inch. If the true total length of the perimeter
-
Consider a reaction mixture containing 100.0 mL of 0.100 M borate buffer at pH = pKa = 9.24. At pH = pKa, we know that [H 3 BO 3 ] = [H 2 BO -3 ] = 0.050 0 M. Suppose that a chemical reaction whose...
-
Calculate the pH of a 0.010 M solution of each amino acid in the form drawn here. H,N* NH2 NH2 NH C=0 CH2 CH2 S CH2 CH2 CH2 CH2 (a) HCHCO, (b) H3CHCO, (c) H,NCHCO, Glutamine Cysteine Arginine
-
A researcher decides to see how effective a pain medication is. Eight randomly selected subjects were asked to determine the severity of their pain by using a scale of 1 to 10, with 1 being very...
-
Required information Use the following information for the Exercises below. (Algo) [The following information applies to the questions displayed below.] Ramirez Company installs a computerized...
-
Reproduced below from Farthington Supply s accounting records is the accounts receivable subledger along with selected general ledger accounts. General Ledger Accounts Receivable Dec. 3 1 / 2 2...
-
James A. and Ella R. Polk, ages 70 and 65, respectively, are retired physicians who live at 3319 Taylorcrest Street, Houston, Texas 77079. Their three adult children (Benjamin Polk, Michael Polk, and...
-
Required information [The following information applies to the questions displayed below.] Shauna Coleman is single. She is employed as an architectural designer for Streamline Design (SD). Shauna...
-
The following are the ratings of men by women in an experiment involving speed dating. Use the given data to construct a boxplot and identify the 5-number summary. 3.0 3.5 4.0 4.5 5.5 5.5 6.5 6.5 6.5...
-
Sandra, a single taxpayer, has taxable income of $94,355. Using the tax tables, she has determined that her tax liability is: a. $16,825. b. $16,820. c. $12,480. d. $12,475.
-
Grace is training to be an airplane pilot and must complete five days of flying training in October with at least one day of rest between trainings. How many ways can Grace schedule her flying...
-
For a silver-silver chloride electrode, the following potentials are observed: E = 0.222 V E(saturated KCl) = 0.197 V From these potentials, find the activity of Cl - in saturated KCl. Calculate E...
-
A cell was prepared by dipping a Cu wire and a saturated calomel electrode into 0.10 M CuSO 4 solution. The Cu wire was attached to the positive terminal of a potentiometer and the calomel electrode...
-
Explain why a silver electrode can be an indicator electrode for Ag + and for halides.
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


