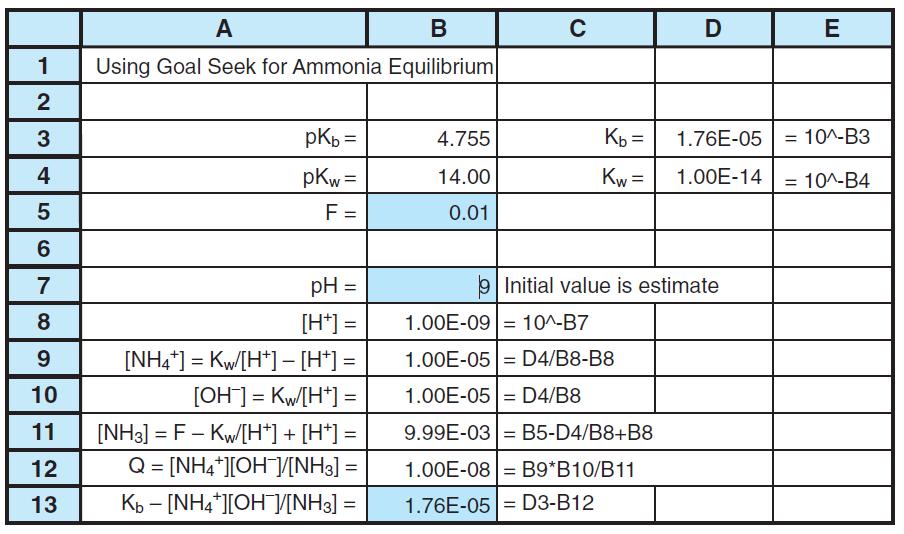
Modify Figure 8-7 to find the concentrations of species in 0.05 M NH3. The only change required
Question:
Modify Figure 8-7 to find the concentrations of species in 0.05 M NH3. The only change required is the value of F. How do the pH and fraction of ammonia hydrolysis (= [NH+4 ]/([NH+4 ] + [NH3])) change when the formal concentration of NH3 increases from 0.01 to 0.05 M?
Transcribed Image Text:
А В C E Using Goal Seek for Ammonia Equilibrium pKb = 4.755 Kb = 1.76E-05 = 10^-B3 4 pKw = 14.00 Kw = 1.00E-14 10^-B4 %3D F = 0.01 7 pH = Initial value is estimate 8 [H*] = 1.00E-09 10^-B7 9. [NH4*] = Kw/[H*] - [H*] = 1.00E-05= D4/B8-B8 10 [OH] = Kw[H*] = 1.00E-05 = D4/B8 [NH3] = F - Kw[H*] + [H*] = Q = [NH4*][OHV[NH3] = 11 9.99E-03 = B5-D4/B8+B8 12 1.00E-08 = B9*B10/B11 %3D 13 Kb - [NH4*][OH]/V[NH3] = 1.76E-05= D3-B12 2. LO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (14 reviews)
pH changes from 10 to 9 at the equivalence point Fraction of NH3 hydrolysis changes from 06001 to 04...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
Find the concentrations of Cu2+ (aq), NH3 (aq), and [Cu(NH3)4]2+(aq) at equilibrium when 0.10 mol Cu2+(aq) and 0.40 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for...
-
Find the concentrations of Ag+(aq), NH3(aq), and [Ag(NH3)2]+(aq) at equilibrium when 0.10 mol Ag+(aq) and 0.10 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for the...
-
The University of Professional Studies, Accra (UPSA) is a public university in Ghana. UPSA is the first university in Ghana to provide both academic and business professional education. The...
-
Repeat Exercise 1b, substituting respondent's social class (CLASS) as the independent variable in separate models for men and women. What can you conclude about the relationship between CLASS and...
-
The acceleration of a particle is defined by the relation a = 12x 28, where a and x are expressed in m/s2 and meters, respectively. Knowing that v = 8m/s when x = 0, determine (a) The maximum value...
-
Identify espoused values that are appealing and ask how they are implemented within the organization
-
1. If you were the CEO of US Airways, what would you do to confront the competition from its low-cost competitors? 2. Can US Airways survive by remaining the same carrier it is today? 3. If you were...
-
Use this information to answer the following questions: The following information is taken from the 2015 annual report to shareholders of Hewlett-Packard (HP) Co. Provision for doubtful accounts 2014...
-
The unadjusted trial balance that you prepared for PS Music at the end of Chapter 2 should appear as follows: PS Music UNADJUSTED TRIAL BALANCE July 31, 2016 ACCOUNT TITLE DEBIT CREDIT 1 Cash...
-
A 40.0-mL solution of 0.040 0 M Hg 2 (NO 3 ) 2 was titrated with 60.0 mL of 0.100 M KI to precipitate Hg 2 I 2 (K sp = 4.6 10 -29 ). (a) Show that 32.0 mL of KI are needed to reach the equivalence...
-
Find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: (a) Look up the equilibrium constants in the appendixes and write their pK values. The...
-
When are adjusting entries made to the worksheet, and what is their purpose? When are the corresponding voucher entries made?
-
BREAD Products' pretax income for 2019 is * (1 Point) BREAD Products has no Work in Process or Finished Goods inventories at the close of business on December 31, 2018. The balances of BREAD's...
-
Convert the following line of code into assembly language. A (A B)+(BA) Where A and B are both 8-bit variables Activate Windows
-
14. Create a one variable Data Table from what you just copied and pasted giving the total sales for each department, and the Largest Sale from each department. Start your Criteria range in cell A1....
-
E4.1 (LO 1), C The following independent situations require professional judgment for determining when to recognize revenue from the transactions. a. Southwest Airlines sells you an advance-purchase...
-
Spring Flings Company, a fashion retailer that specializes in colorful graphic tees, prepares a master budget on a quarterly basis. The company has assembled the following data to assist in preparing...
-
Determine the tax liability, marginal tax rate, and average tax rate (rounded to two decimal places) in each of the following cases. Use the tax tables to determine tax liability. a. Married...
-
After graduating from college and working a few years at a small technology firm. Preet scored a high-level job in the logistics department at Amex Corporation. Amex sells high-quality electronic...
-
What does the selectivity coefficient tell us? Is it better to have a large or a small selectivity coefficient?
-
Suppose that the silver-silver chloride electrode in Figure 14-2 is replaced by a saturated calomel electrode. Calculate the cell voltage if [Fe 2+ ] / [Fe 3+ ] = 2.5 10 -3 . Figure 14-2
-
Why is it preferable to use a metal ion buffer to achieve pM = 8 rather than just dissolving enough M to give a 10-8 M solution?
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


