Question: From standard reduction potentials, which of the following metals would you expect to dissolve in HCl HCl by the reaction ''[].. Zn, Fe, Co, Al,
From standard reduction potentials, which of the following metals would you expect to dissolve in HCl HCl by the reaction •''•[].. Zn, Fe, Co, Al, Hg, Cu, Pt, Au?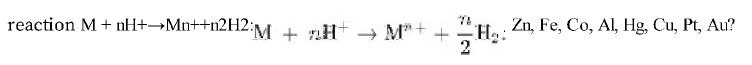 Zn, Fe, Co, Al, Hg, Cu, Pt, Au? (When the potential predicts that the element will not dissolve, it probably will not. If it is expected to dissolve, it may dissolve if some other process does not interfere. Predictions based on standard reduction potentials at 25°C25°C are only tentative, because the potentials and activities in hot, concentrated solutions vary widely from those in the table of standard potentials.)
Zn, Fe, Co, Al, Hg, Cu, Pt, Au? (When the potential predicts that the element will not dissolve, it probably will not. If it is expected to dissolve, it may dissolve if some other process does not interfere. Predictions based on standard reduction potentials at 25°C25°C are only tentative, because the potentials and activities in hot, concentrated solutions vary widely from those in the table of standard potentials.)
reaction M + nH+Mn++n2H2M + H+ M+ + 2 H: Zn, Fe, Co, Al, Hg, Cu, Pt, Au?
Step by Step Solution
3.29 Rating (173 Votes )
There are 3 Steps involved in it
From the standard reduction potentials the following metals are expected ... View full answer

Get step-by-step solutions from verified subject matter experts


