Question
1. Should the chromatography serve to purify the sample? 2. Explain the formation of isomer Post-Lab Discussion/Analysis 1. Compare the TLC plates for the crude
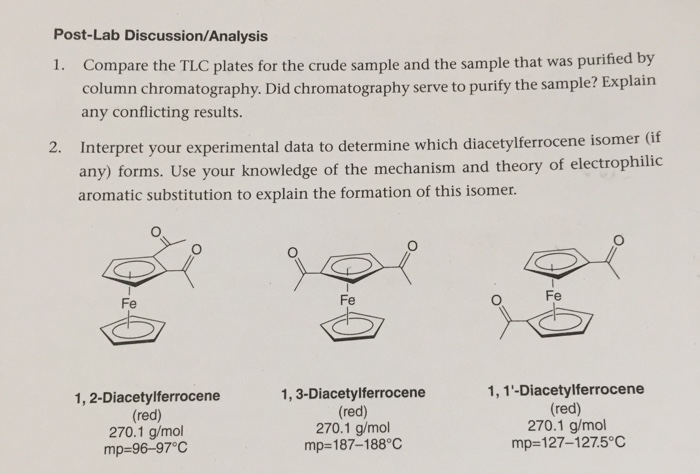
Post-Lab Discussion/Analysis 1. Compare the TLC plates for the crude sample and the sample that was purified by column chromatography. Did chromatography serve to purify the sample? Explain any conflicting results. 2. Interpret your experimental data to determine which diacetylferrocene isomer (if any) forms. Use your knowledge of the mechanism and theory of electrophilic aromatic substitution to explain the formation of this isomer. - Fe 1,2-Diacetylferrocene (red) 270.1 g/mol mp=96-97C Fe 1,3-Diacetylferrocene (red) 270.1 g/mol mp=187-188C Fe 1, 1'-Diacetylferrocene (red) 270.1 g/mol mp=127-127.5C
Step by Step Solution
3.48 Rating (178 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Andersons Business Law and the Legal Environment
Authors: David P. Twomey, Marianne M. Jennings
22nd edition
978-113358758, 1133587585, 978-1133587583
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



