Question
19. Write the balanced equation for the following reactions i. Substitution reaction between methane and chlorine ii. Esterification reaction between ethanoic acid and ethanol iii.
19. Write the balanced equation for the following reactions
i. Substitution reaction between methane and chlorine
ii. Esterification reaction between ethanoic acid and ethanol
iii. Addition reaction between propene and hydrogen chloride
iv. Elimination reaction between 2-bromobutane and methoxi(CH3O−)
20. What product(s) would you expect from sulfonation of the following compounds?
i. Nitrobenzene
ii. Bromobenzene
iii. Toluene
iv. Benzoic acid
v. Benzonitrile
21. Draw resonance structures of the three possible carbocation intermediates to show how a
methoxyl group (−OCH3) directs bromination toward ortho and para positions.
22. Tell whether each of the following reactions is likely to be SN1, SN2, E1, or E2:
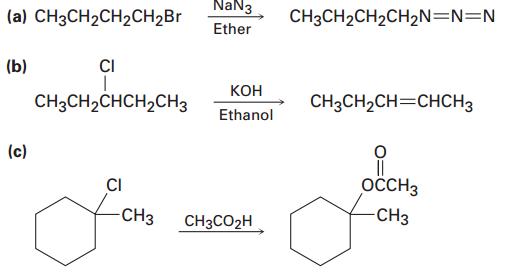
23. Which of the following SN2 reactions would you expect to be faster?
(a) Reaction of CN (cyanide ion) with CH3CH(Br)CH3 or with CH3CH2CH2Br?
(b) Reaction of I with (CH3)2CHCH2Cl or with H2C=CHCl?
25. Write the structure of the organic product on the nitration of each of the following
i. p-Methylbenzoic acid
ii. m-Dichlorobenzene
iii. m-Dinitrobenzene
iv. p-Methoxyacetophenone
(a) CH3CH2CH2CH2B. NaN3 CH3CH2CH2CH2N=N=N Ether (b) CI CH3CH2CHCH2CH3 CH3CH2CH=CHCH3 Ethanol (c) || OCCH3 CI -CH3 CH3CO2H CH3
Step by Step Solution
3.48 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


