Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gasifier is used to gasify glucose with air at a temperature of X1[C] and pressure of X2[MPa]. Assuming no tar-ash production, and given the
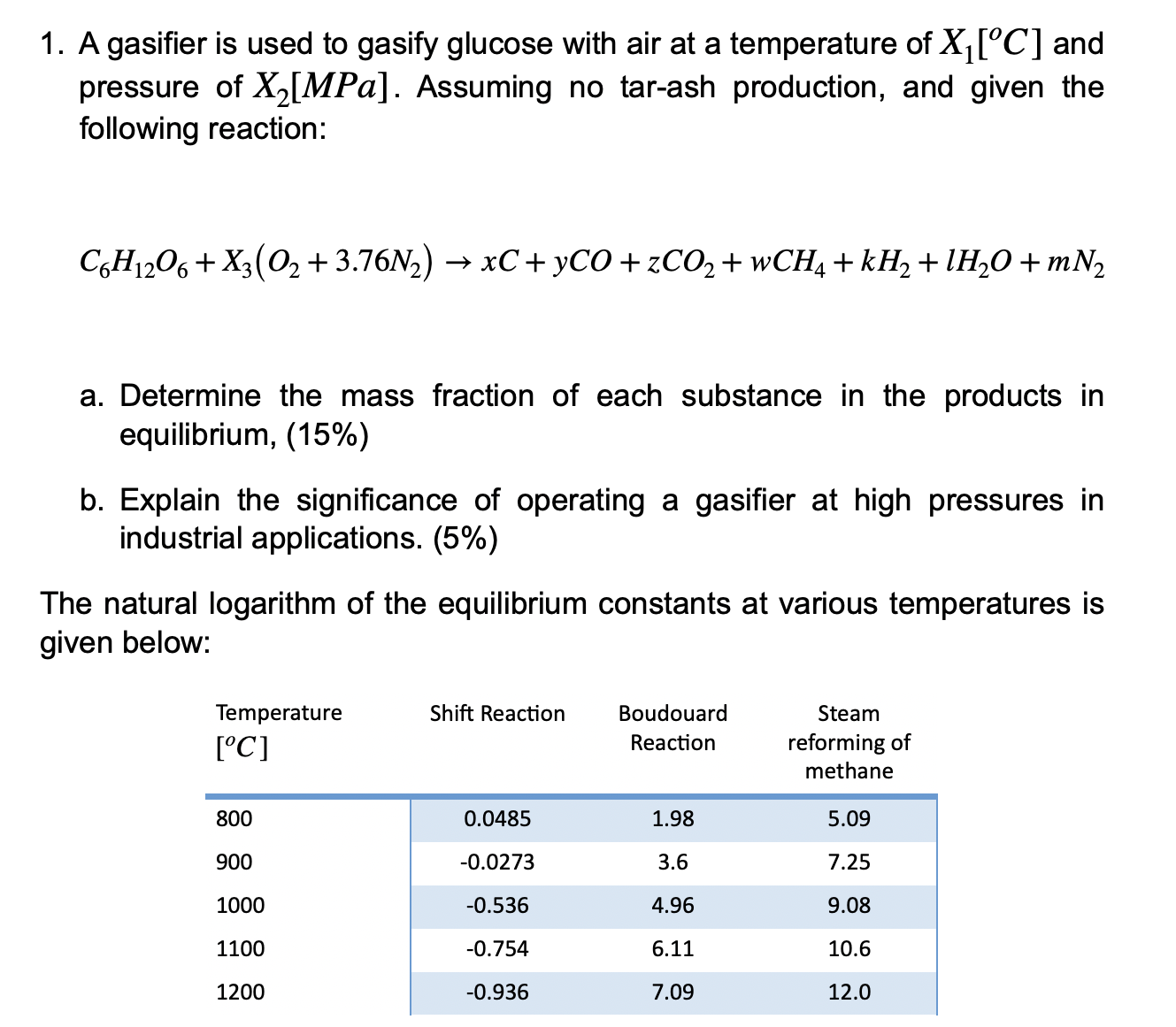 A gasifier is used to gasify glucose with air at a temperature of X1[C] and pressure of X2[MPa]. Assuming no tar-ash production, and given the following reaction: C6H12O6+X3(O2+3.76N2)xC+yCO+zCO2+wCH4+kH2+lH2O+mN2 a. Determine the mass fraction of each substance in the products in equilibrium, (15\%) b. Explain the significance of operating a gasifier at high pressures in industrial applications. (5%) The natural logarithm of the equilibrium constants at various temperatures is jiven below
A gasifier is used to gasify glucose with air at a temperature of X1[C] and pressure of X2[MPa]. Assuming no tar-ash production, and given the following reaction: C6H12O6+X3(O2+3.76N2)xC+yCO+zCO2+wCH4+kH2+lH2O+mN2 a. Determine the mass fraction of each substance in the products in equilibrium, (15\%) b. Explain the significance of operating a gasifier at high pressures in industrial applications. (5%) The natural logarithm of the equilibrium constants at various temperatures is jiven below Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


