Question: H3PO4 is a weak triprotic acid. The pH of 0.1M Naz H PO, (aq) is calculated by (A) PKa, + PKoz] (B) PK, +
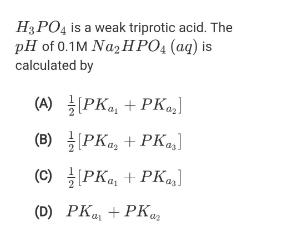
H3PO4 is a weak triprotic acid. The pH of 0.1M Naz H PO, (aq) is calculated by (A) PKa, + PKoz] (B) PK, + PK,s] (C) PK + PKas] (D) PK + PKar
Step by Step Solution
3.56 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


