Question
Calculate the change in Gibbs free energy for each of the following sets of ?Hrxn, ?Srxn, and T. 1. ?Harxn=81 kj, ?Srxn= 144 J/K,
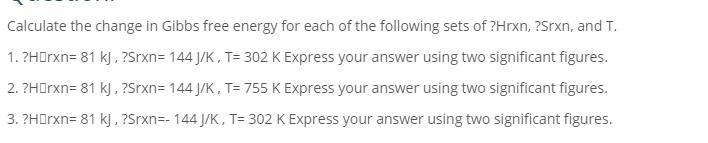
Calculate the change in Gibbs free energy for each of the following sets of ?Hrxn, ?Srxn, and T. 1. ?Harxn=81 kj, ?Srxn= 144 J/K, T= 302 K Express your answer using two significant figures. 2. ?Harxn= 81 kJ, ?Srxn=144 J/K, T= 755 K Express your answer using two significant figures. 3. ?HOrxn= 81 kj, ?Srxn=-144 J/K, T= 302 K Express your answer using two significant figures.
Step by Step Solution
3.52 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
1 2 AG RX AGRIN AGRAN AGRAN AH RYN AGRYW T AS ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Precalculus
Authors: Michael Sullivan
9th edition
321716835, 321716833, 978-0321716835
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



