Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you please help me with b? i am having problem with regards simplifying the kc equation to solve for x and using the quadratic
can you please help me with b? i am having problem with regards simplifying the kc equation to solve for x and using the quadratic formula, a step by step solution would be greatly appreciated 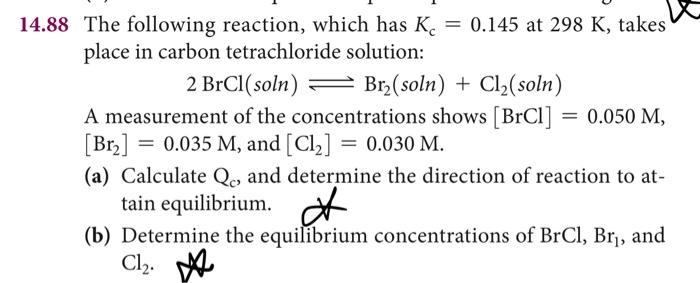
4.88 The following reaction, which has Kc=0.145 at 298K, takes place in carbon tetrachloride solution: 2BrCl(soln)Br2(soln)+Cl2(soln) A measurement of the concentrations shows [BrCl]=0.050M, [Br2]=0.035M, and [Cl2]=0.030M. (a) Calculate Qc, and determine the direction of reaction to attain equilibrium. (b) Determine the equilibrium concentrations of BrCl1,Br1, and Cl2M 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


