Answered step by step
Verified Expert Solution
Question
1 Approved Answer
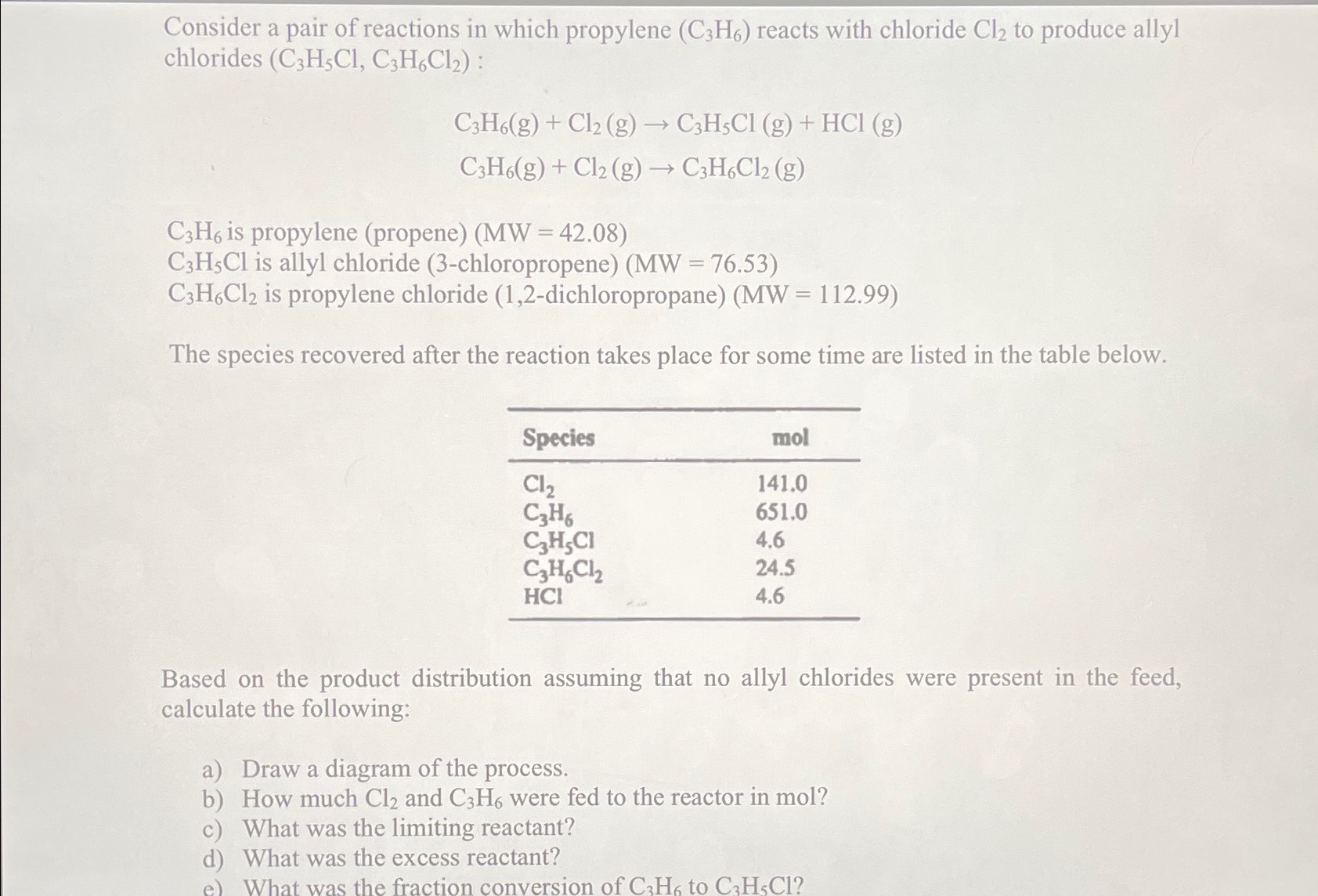
Consider a pair of reactions in which propylene ( C 3 H 6 ) reacts with chloride C l 2 to produce allyl chlorides (
Consider a pair of reactions in which propylene reacts with chloride to produce allyl chlorides :
is propylene propene
is allyl chloride chloropropene
is propylene chloride dichloropropane
The species recovered after the reaction takes place for some time are listed in the table below.
tableSpeciesmol
Based on the product distribution assuming that no allyl chlorides were present in the feed, calculate the following:
a Draw a diagram of the process.
b How much and were fed to the reactor in mol?
c What was the limiting reactant?
d What was the excess reactant?
e What was the fraction conversion of to
f What was the selectivity of CHCl relative to CHCl
g What was the yield of CHCl expressed in g of CHCl expressed in g of CHCl to the g of CH fed to the reactor?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


