Answered step by step
Verified Expert Solution
Question
1 Approved Answer
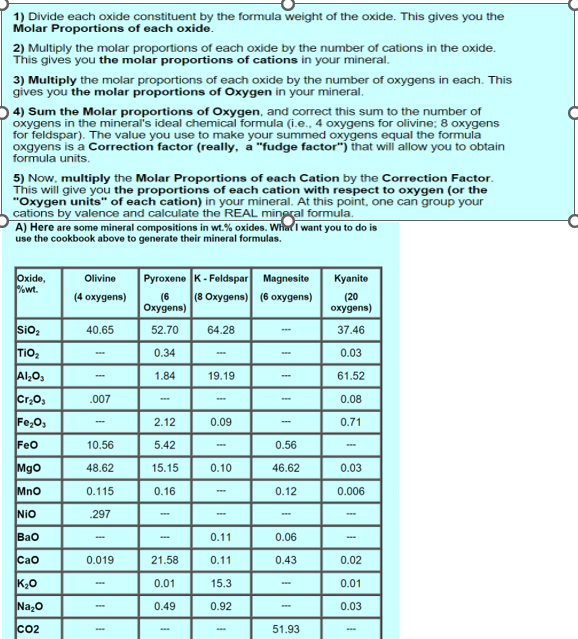
Divide each oxide constituent by the formula weight of the oxide. This gives you the Molar Proportions of each oxide. Multiply the molar proportions of
Divide each oxide constituent by the formula weight of the oxide. This gives you the Molar Proportions of each oxide.
Multiply the molar proportions of each oxide by the number of cations in the oxide. This gives you the molar proportions of cations in your mineral.
Multiply the molar proportions of each oxide by the number of oxygens in each. This gives you the molar proportions of Oxygen in your mineral.
Sum the Molar proportions of Oxygen, and correct this sum to the number of oxygens in the mineral's ideal chemical formula ie oxygens for olivine; oxygens for feldspar The value you use to make your summed oxygens equal the formula oxgyens is a Correction factor really a "fudge factor" that will allow you to obtain formula units.
Now, multiply the Molar Proportions of each Cation by the Correction Factor. This will give you the proportions of each cation with respect to oxygen or the "Oxygen units" of each cation in your mineral. At this point, one can group your cations by valence and calculate the REAL mingral formula.
A Here are some mineral compositions in wt oxides. Whina I want you to do is use the cookbook above to generate their mineral formulas.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


