Answered step by step
Verified Expert Solution
Question
1 Approved Answer
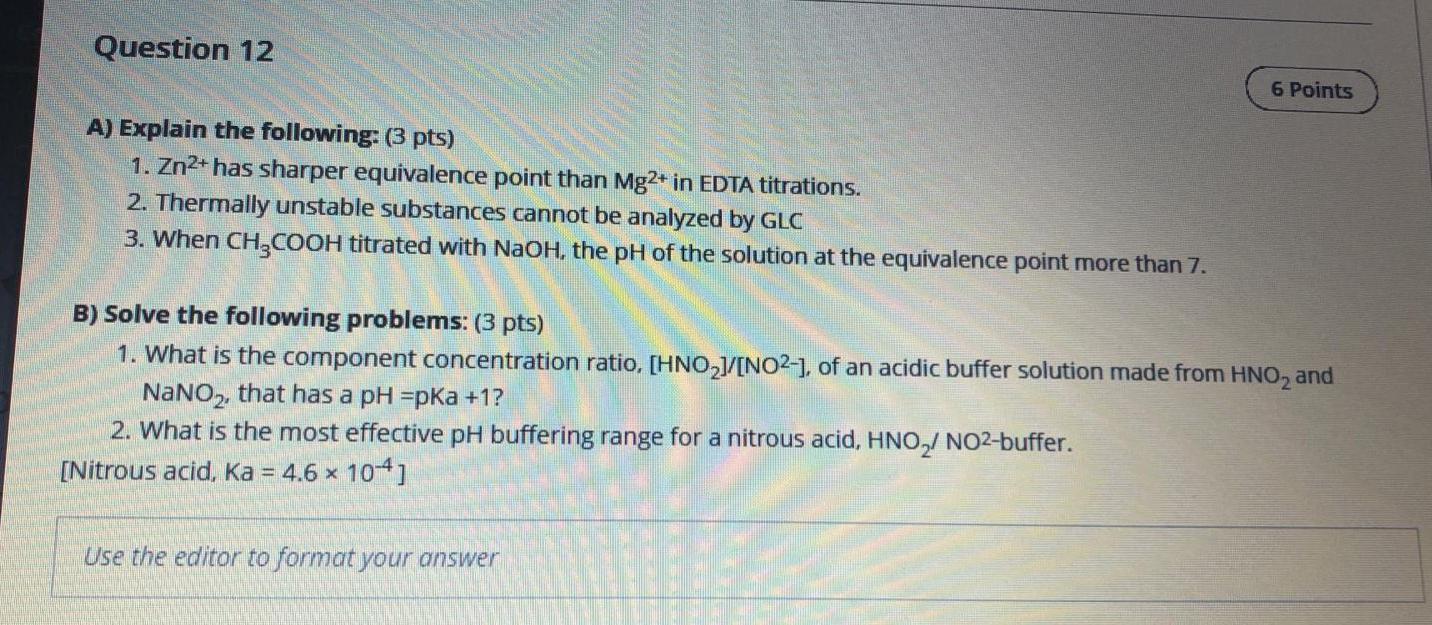
I want it now please Question 12 6 Points A) Explain the following: (3 pts) 1. Zn2+ has sharper equivalence point than Mg2+ in EDTA
I want it now please
Question 12 6 Points A) Explain the following: (3 pts) 1. Zn2+ has sharper equivalence point than Mg2+ in EDTA titrations. 2. Thermally unstable substances cannot be analyzed by GLC 3. When CH3COOH titrated with NaOH, the pH of the solution at the equivalence point more than 7. B) Solve the following problems: (3 pts) 1. What is the component concentration ratio, [HNO2J/[NO2-), of an acidic buffer solution made from HNO2 and NaNO2, that has a pH =pKa +1? 2. What is the most effective pH buffering range for a nitrous acid, HNO, NO2-buffer. [Nitrous acid, Ka = 4.6 x 104] X Use the editor to format yourStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


