Question
Match the term with its definition. Match the description with its meaning. Compound that is electron pair acceptor is called A substance that can
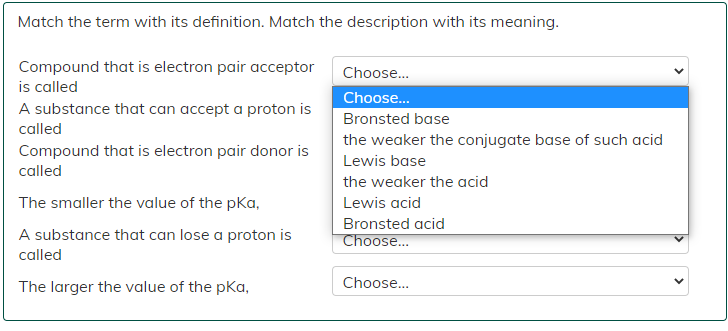
Match the term with its definition. Match the description with its meaning. Compound that is electron pair acceptor is called A substance that can accept a proton is called Compound that is electron pair donor is called The smaller the value of the pka, A substance that can lose a proton is called The larger the value of the pKa, Choose... Choose... Bronsted base the weaker the conjugate base of such acid Lewis base the weaker the acid Lewis acid Bronsted acid Choose... Choose...
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Compound that is electron pair acceptor is called Lewis acid A substance ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Accounting
Authors: Robert Kemp, Jeffrey Waybright
2nd edition
978-0132771801, 9780132771580, 132771802, 132771586, 978-0133052152
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



