Question
Part 1 Run 1 (NaOH+HCl): mass= 100.34g c=4.184 dT=7.2Kelvin Part 1 Run 2 (NaOH+HCl) mass=101.72g c=4.184 dT=6.9Kelvin Part 2 Run 1 (Acetic Acid+NaOH) mass=100.94g dT=6.9kelvin
Part 1 Run 1 (NaOH+HCl): mass= 100.34g c=4.184 dT=7.2Kelvin Part 1 Run 2 (NaOH+HCl) mass=101.72g c=4.184 dT=6.9Kelvin
Part 2 Run 1 (Acetic Acid+NaOH) mass=100.94g dT=6.9kelvin Part 2 Run 2 (Acetic Acid+NaOH) mass=100.38g dT=6.3Kelvin
Concentration of NaOH: 1.00M
Concentration HCl:0.050L
Concentration of Acetic Acid: 1.02M
1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate qcontents and qsystem. 2. Calculate the moles of H2O (aq.) produced, nrxn. Be sure to identify the limiting reagent. 3. Calculate the standard molar enthalpy change, H0 rxn, for reaction (2.8). 4. CHEM 1020: Calculate the average H0 rxn from the two runs.
Calculation of delta H0rxn for Part II 1. Again, assume the heat capacity of the final solution is 4.184 J K-1 g-1. Calculate qcontents and qsystem. 2. Calculate the number of moles of NaCH3COO(aq.) produced, nrxn. Again, identify the limiting reagent. 3. Calculate the standard molar enthalpy change for reaction (2.9) for each of the two runs. 4. CHEM 1020: Calculate the average deltaH0rxn from the two runs.
1. Why should deltaH0 rxn for reaction (acetic+naoh) be less exothermic than delta H0 rxn for reaction (NaOH+HCl)? 2. Using Hesss Law and your results from parts I and II, calculate deltaH0 rxn, for reaction (CH3COOH(aq) --> CH3COO-(aq)+H+(aq) 3. Calculate the delta H0f of the OH- ion given that the delta H0 f (H2O) = -285.8 kJ mol-1. 4. Imagine an endothermic reaction had been studied (i.e. deltaH0rxn > 0). In this experiment we assumed the calorimeter was perfect (qcalorimeter = 0). If, however, the calorimeter were not perfect and heat flowed from the calorimeter to the contents, would the measured delta H0rxn be more or less endothermic than the true deltaH0rxn? 5. Use tabulated standard molar enthalpies of formation (from your textbook) to calculate delta H0 rxn for the following (unbalanced) reactions: a. Al(s) + Fe2O3(s) Fe(s) + Al2O3(s) b. PbO(s) + C(s) CO(g) + Pb(s) c. CaCO3(s) CO2(g) + CaO(s) 6. When 1.464 g of propane (C3H8(g)) are burned in an excess of oxygen at 1 bar and 298.15 K, 67.9 kJ of heat are generated. The products of the combustion are gaseous water and carbon dioxide. a. Write a balanced chemical reaction for the combustion of propane. b. If delta Hf0(C3H8(g)) = -103.9 kJ mol-1 and delta Hf0(H2O(g)) = -241.8 kJ mol-1, calculate deltaHf0(CO2(g)). c. How much heat is released per kilogram of carbon dioxide produced?
dT= Delta Temperature, the weird A.. symbols are the delta sign, Please Help
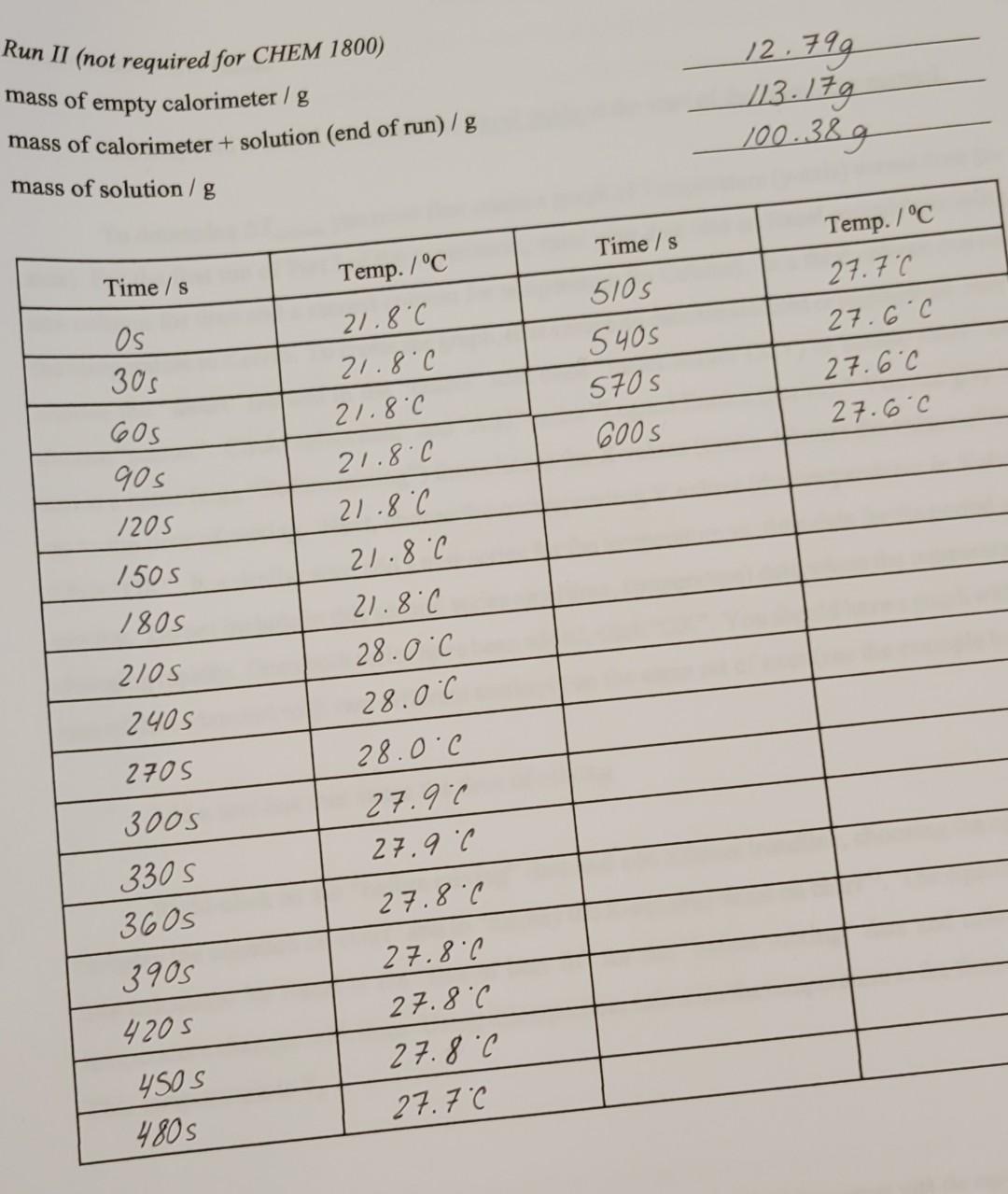
values needed to solve the question are all CLEARLY stated at the top of the question. please re-read
Run II (not required for CHEM 1800) mass of empty calorimeter / g mass of calorimeter + solution (end of run) / g mass of solution / gStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


