Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer fully. Thank you! 1. Draw a Newman Projection for each of the molecules below along the indicated bond with the indicated perspective. Make
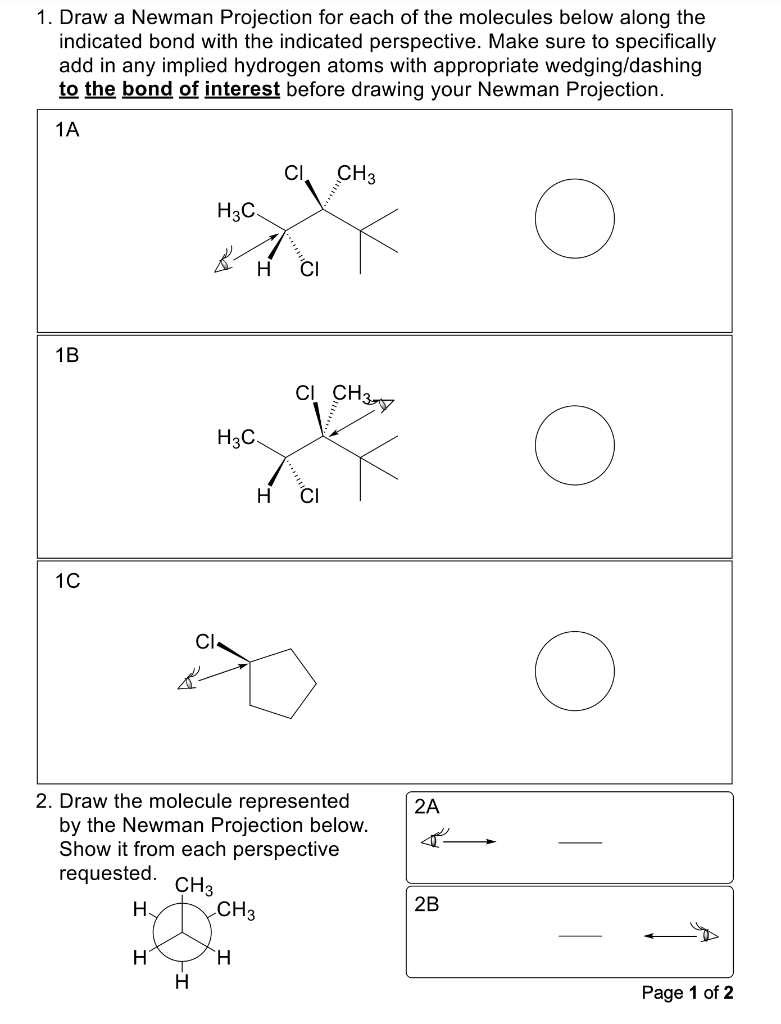
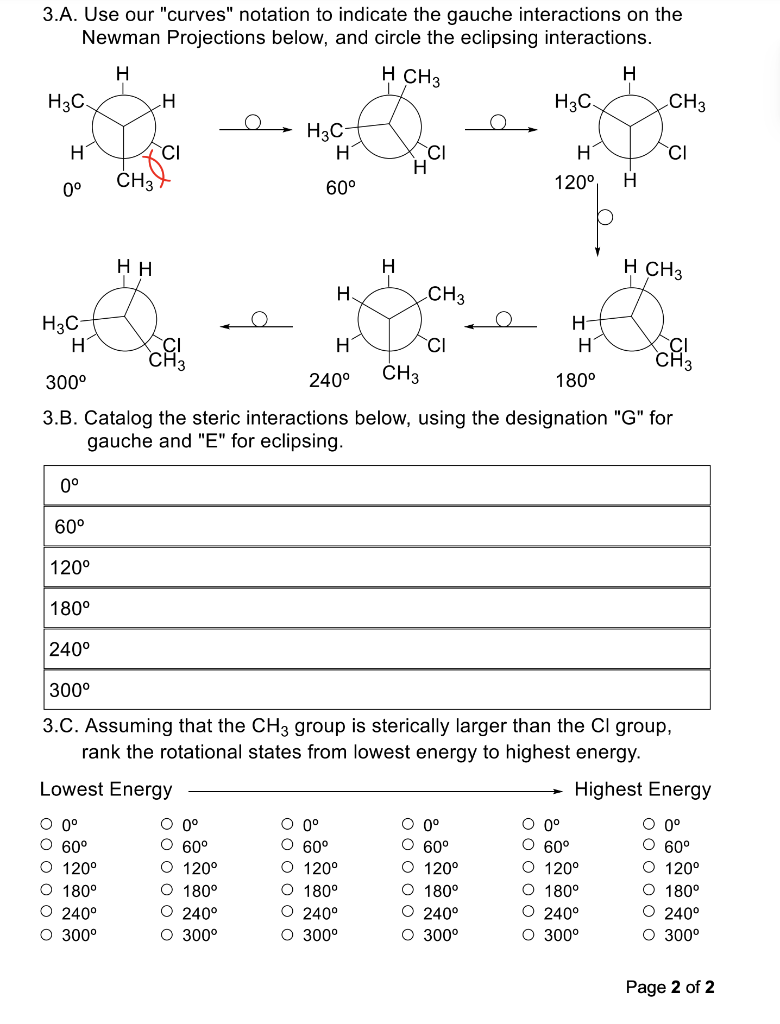
Please answer fully. Thank you!
1. Draw a Newman Projection for each of the molecules below along the indicated bond with the indicated perspective. Make sure to specifically add in any implied hydrogen atoms with appropriate wedging/dashing to the bond of interest before drawing your Newman Projection. 1A 1B 1C 2. Draw the molecule represented by the Newman Projection below. Show it from each perspective requestan 2B Page 1 of 2 3.A. Use our "curves" notation to indicate the gauche interactions on the Newman Projections below, and circle the eclipsing interactions. 300 3.B. Catalog the steric interactions below, using the designation "G" for gauche and "E" for eclipsing. 3.C. Assuming that the CH3 group is sterically larger than the Cl group, rank the rotational states from lowest energy to highest energy. \begin{tabular}{clllll} Lowest Energy & & & & \multicolumn{2}{c}{ Highest Energy } \\ \cline { 2 - 6 } 0 & 0 & 0 & 0 & 0 & 0 \\ 60 & 60 & 60 & 60 & 60 & 60 \\ 120 & 120 & 120 & 120 & 120 & 120 \\ 180 & 180 & 180 & 180 & 180 & 180 \\ 240 & 240 & 240 & 240 & 240 & 240 \\ 300 & 300 & 300 & 300 & 300 & 300 \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


