Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help! 0 1. When heated to 800 K, cyclobutene decomposes to ethene according to the reaction C.Hace 2 CH4g). Data for this reaction is
please help! 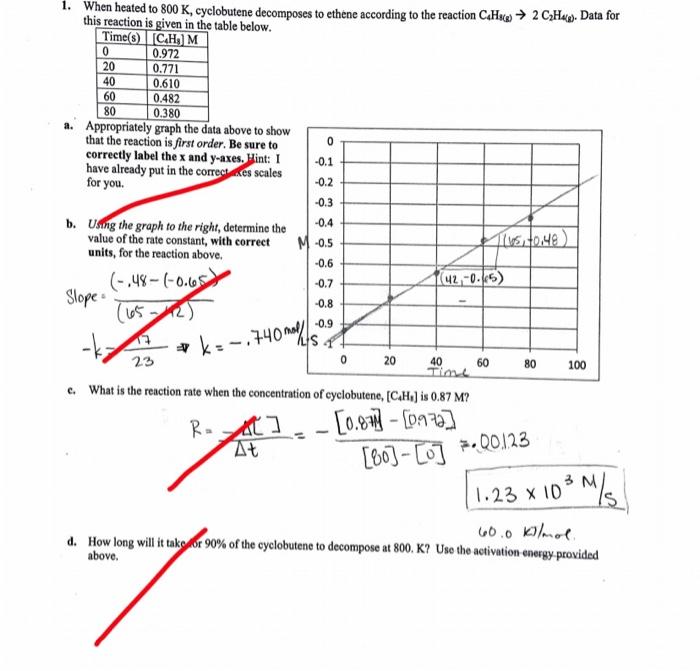
0 1. When heated to 800 K, cyclobutene decomposes to ethene according to the reaction C.Hace 2 CH4g). Data for this reaction is given in the table below. Time(s)[CH] M 0 0.972 20 0.771 40 0.610 60 0.482 80 0.380 a. Appropriately graph the data above to show that the reaction is first order. Be sure to correctly label the x and y-axes. Hint: 1 -0.1 have already put in the correctemes scales for you. -0.2 -0.3 b. Using the graph to the right, determine the -0.4 value of the rate constant, with correct M -0.5 Tuto.me units, for the reaction above. -0.6 (-.48-(-0.65) -0.7 (42,-0.65) Slope -0.8 -0.9 ALS 23 0 20 40 60 80 100 Tin c. What is the reaction rate when the concentration of cyclobutene, [C.H.) is 0.87 M? R - [0.87 [0.972] At () *k:-.740 mall - [80] - [o] 7.00123 1.23 x 103 M/S 60.0 kilmol d. How long will it tako or 90% of the cyclobutene to decompose at 800. K? Uso the activation energy provided above. -7 0 1. When heated to 800 K, cyclobutene decomposes to ethene according to the reaction C.Hace 2 CH4g). Data for this reaction is given in the table below. Time(s)[CH] M 0 0.972 20 0.771 40 0.610 60 0.482 80 0.380 a. Appropriately graph the data above to show that the reaction is first order. Be sure to correctly label the x and y-axes. Hint: 1 -0.1 have already put in the correctemes scales for you. -0.2 -0.3 b. Using the graph to the right, determine the -0.4 value of the rate constant, with correct M -0.5 Tuto.me units, for the reaction above. -0.6 (-.48-(-0.65) -0.7 (42,-0.65) Slope -0.8 -0.9 ALS 23 0 20 40 60 80 100 Tin c. What is the reaction rate when the concentration of cyclobutene, [C.H.) is 0.87 M? R - [0.87 [0.972] At () *k:-.740 mall - [80] - [o] 7.00123 1.23 x 103 M/S 60.0 kilmol d. How long will it tako or 90% of the cyclobutene to decompose at 800. K? Uso the activation energy provided above. -7 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


