Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please show every steps P2-48 The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane (C), that is, A +
please show every steps 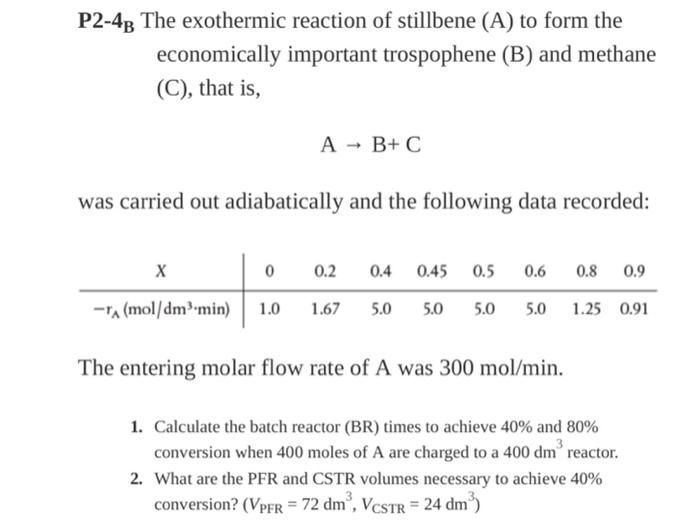
P2-48 The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane (C), that is, A + B+C was carried out adiabatically and the following data recorded: 0 0.2 0.4 0.45 0.5 0.6 0.8 0.9 A (mol/dm min) 1.0 1.67 5.0 5.0 5.0 5.0 1.25 0.91 The entering molar flow rate of A was 300 mol/min. 1. Calculate the batch reactor (BR) times to achieve 40% and 80% conversion when 400 moles of A are charged to a 400 dm reactor. 2. What are the PFR and CSTR volumes necessary to achieve 40% conversion? (VPFR = 72 dm, VCSTR = 24 dm) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


