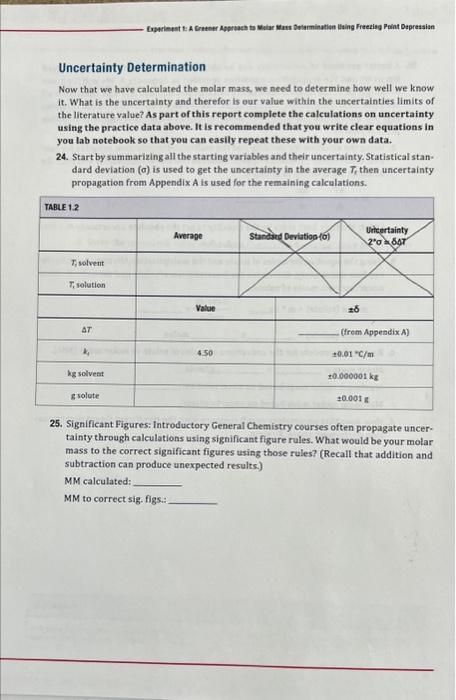
Practice Calculations Use the following data to answer the questions below. Assume 9.045g of stearicacid, and 1.327g of unknown for the mixture runs 4-6. Assume the ki is 4.50Ckg/mole. Assume the uncertainty is 1 in the last digit (0.001 g for the masses and 0.01 for k. . Run1:Run2:Run3:Run4:Run5:Run6:SolventSolventSolventMixture2gMixture2gMixture2gTfremm=68.90.2CTfamm=69.00.2CTfulmet=67.80.2CT2s=64.60.3CT2s=64.30.3CT2s=64.70.3C 22. In groups, create a flow chart below including the equations required to process the raw data into molar mass for the runs given. Indicate when values are averaged and Ts are determined. - calcuiale Aug Tf por solvent - Tf hir ma (sobute + jorvent) - determine aepresion of friting point eor each of 4,5,6 tins - we have, T=ki molaliny using this determine, molaity (mei/key) - form mas of juivent in kg, estimate moles of sotute - for each vun decromine molar maus mujulmole. - huerge mese th fiet thoior mald 23. Using the data above, in your lab notebook set up a table that will clearly show the progression of data from raw data to final molar mass for the data above (see Table 1.2), Note: The masses for your experiment will be different as they will be 2g. Molality for 1.327 grams: .911=.024mm/tg Molar Mass for 1.327 grams: 141.2+4.2. Tnos=64.5t,3=k6xmx2157.0+105.3=161.2m1=(4.50+.01)(6t.6+.2)(64.5+.3)=.977molkg=161.2=157.0=165.3101.2=4.2m2=(4.50.01)(6i6.2)(64.5.3)9.935movlkjm=277+.935=911molkg=.911.807.935.911=.024 Uncertainty Determination Now that we have calculated the molar mass, we need to determine how well we know it. What is the uncertainty and therefor is our value within the uncertainties limits of the literature value? As part of this report complete the calculations on uncertainty using the practice data above. It is recommended that you write clear equations in you lab notebook so that you can easily repeat these with your own data. 24. Start by summarizing all the starting variables and their uncertainty. Statistical standard deviation () is used to get the uncertainty in the average T, then uncertainty propagation from Appendix A is used for the remaining calculations. 25. Significant Figures: Introductory General Chemistry courses often propagate uncertainty through calculations using significant figure rules. What would be your molar mass to the correct significant figures using those rules? (Recall that addition and subtraction can produce unexpected results.) MM calculated: MM to correct sig. figs: 26. A better estimate of uncertainty can be obtained by the combination of standard deviation and the propagation equations provided in Appendix A. We will limit our investigation to the random uncertainty calcalations. Because this next equation is all multiplication and division, this can be done in one equation using all of the variables that are used in the complete calculation or it can be done stepwise. The stepwise process follows. 3. Using the equation for molality and the multiplication/division equation for random uncertainty (Appendix A ) determine the fractional uncertainty and then the absolute uncertainty in molality. m=k4TFrm= (Hint: Multiply both sides by the molality to get the absolute uncertainty mm ) m=moles/kg b. Using the equation for moles of solute and the multiplication/division equation for random uncertainty, determine the fractional uncertainty and then the absolute ancertainty in moles of solute. moles(solate)=mkgmolesmoles=moles(solute)= c. Repeat the process for the conversion of moles of solute to molar mass (MM). Molar mass a g/mole Maximum molar mass based on uncertainties: Minimum molar mass based on uncertainties: d. How does this uncertainty result compare with the significant figure determinathon performed above. Explain. Eaperimeat t: A Greenet Appreach ts Molar Mass Determination Deing Freeting Point Depretsian 27. Summary of Results a. Molar Mass for 1.327 grams: b. Predicted literature molar mass for this practice example: 152.57g/mol C. Relative uncertainty (see Appendix A). Show calculations. d. Does the measured value agree with the literature value? Why or why not? TA Signature Ask your TA to review your work and sign your report. The TA will sign above once satisfied that the student as performed the entire procedure. The report will not be accepted or graded unless signed. Practice Calculations Use the following data to answer the questions below. Assume 9.045g of stearicacid, and 1.327g of unknown for the mixture runs 4-6. Assume the ki is 4.50Ckg/mole. Assume the uncertainty is 1 in the last digit (0.001 g for the masses and 0.01 for k. . Run1:Run2:Run3:Run4:Run5:Run6:SolventSolventSolventMixture2gMixture2gMixture2gTfremm=68.90.2CTfamm=69.00.2CTfulmet=67.80.2CT2s=64.60.3CT2s=64.30.3CT2s=64.70.3C 22. In groups, create a flow chart below including the equations required to process the raw data into molar mass for the runs given. Indicate when values are averaged and Ts are determined. - calcuiale Aug Tf por solvent - Tf hir ma (sobute + jorvent) - determine aepresion of friting point eor each of 4,5,6 tins - we have, T=ki molaliny using this determine, molaity (mei/key) - form mas of juivent in kg, estimate moles of sotute - for each vun decromine molar maus mujulmole. - huerge mese th fiet thoior mald 23. Using the data above, in your lab notebook set up a table that will clearly show the progression of data from raw data to final molar mass for the data above (see Table 1.2), Note: The masses for your experiment will be different as they will be 2g. Molality for 1.327 grams: .911=.024mm/tg Molar Mass for 1.327 grams: 141.2+4.2. Tnos=64.5t,3=k6xmx2157.0+105.3=161.2m1=(4.50+.01)(6t.6+.2)(64.5+.3)=.977molkg=161.2=157.0=165.3101.2=4.2m2=(4.50.01)(6i6.2)(64.5.3)9.935movlkjm=277+.935=911molkg=.911.807.935.911=.024 Uncertainty Determination Now that we have calculated the molar mass, we need to determine how well we know it. What is the uncertainty and therefor is our value within the uncertainties limits of the literature value? As part of this report complete the calculations on uncertainty using the practice data above. It is recommended that you write clear equations in you lab notebook so that you can easily repeat these with your own data. 24. Start by summarizing all the starting variables and their uncertainty. Statistical standard deviation () is used to get the uncertainty in the average T, then uncertainty propagation from Appendix A is used for the remaining calculations. 25. Significant Figures: Introductory General Chemistry courses often propagate uncertainty through calculations using significant figure rules. What would be your molar mass to the correct significant figures using those rules? (Recall that addition and subtraction can produce unexpected results.) MM calculated: MM to correct sig. figs: 26. A better estimate of uncertainty can be obtained by the combination of standard deviation and the propagation equations provided in Appendix A. We will limit our investigation to the random uncertainty calcalations. Because this next equation is all multiplication and division, this can be done in one equation using all of the variables that are used in the complete calculation or it can be done stepwise. The stepwise process follows. 3. Using the equation for molality and the multiplication/division equation for random uncertainty (Appendix A ) determine the fractional uncertainty and then the absolute uncertainty in molality. m=k4TFrm= (Hint: Multiply both sides by the molality to get the absolute uncertainty mm ) m=moles/kg b. Using the equation for moles of solute and the multiplication/division equation for random uncertainty, determine the fractional uncertainty and then the absolute ancertainty in moles of solute. moles(solate)=mkgmolesmoles=moles(solute)= c. Repeat the process for the conversion of moles of solute to molar mass (MM). Molar mass a g/mole Maximum molar mass based on uncertainties: Minimum molar mass based on uncertainties: d. How does this uncertainty result compare with the significant figure determinathon performed above. Explain. Eaperimeat t: A Greenet Appreach ts Molar Mass Determination Deing Freeting Point Depretsian 27. Summary of Results a. Molar Mass for 1.327 grams: b. Predicted literature molar mass for this practice example: 152.57g/mol C. Relative uncertainty (see Appendix A). Show calculations. d. Does the measured value agree with the literature value? Why or why not? TA Signature Ask your TA to review your work and sign your report. The TA will sign above once satisfied that the student as performed the entire procedure. The report will not be accepted or graded unless signed