Question
For the following substances, give the electron pair geometry, molecular geometry, and hybridization of the central atom(s). Put your answer in the following format;
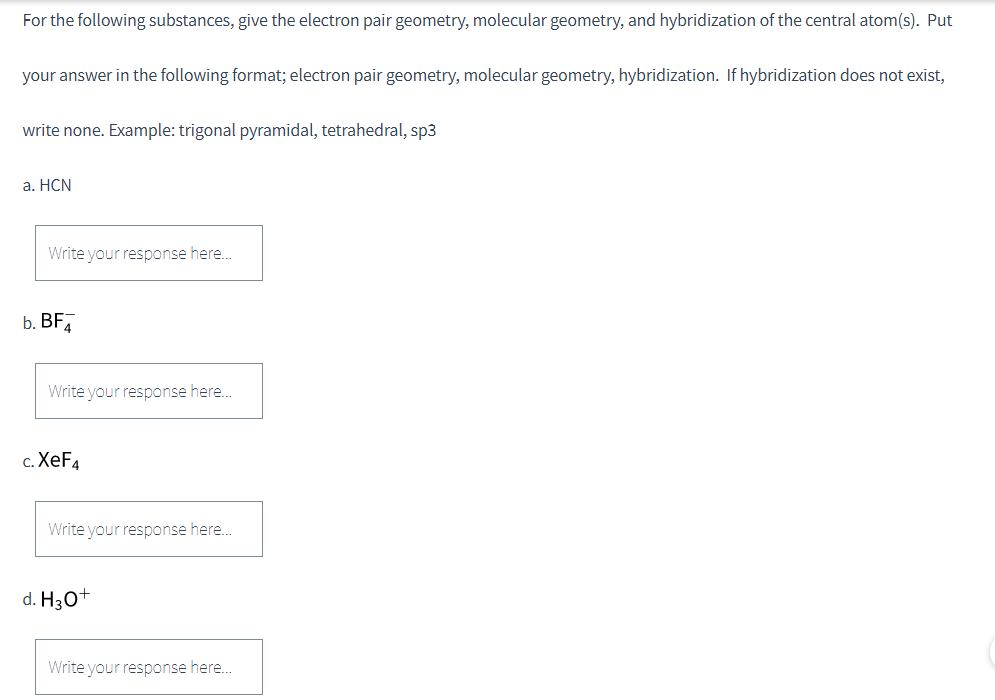
For the following substances, give the electron pair geometry, molecular geometry, and hybridization of the central atom(s). Put your answer in the following format; electron pair geometry, molecular geometry, hybridization. If hybridization does not exist, write none. Example: trigonal pyramidal, tetrahedral, sp3 a. HCN Write your response here... b. BF Write your response here... c. XeF4 Write your response here... d. H3O+ Write your response here...
Step by Step Solution
3.53 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Answers a HCN Electron pair geometry Linear Molecular geometry Linear ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Accounting A Contemporary Approach
Authors: David Haddock, John Price, Michael Farina
2nd edition
73396958, 978-0077630461, 77630467, 978-0073396958
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



