Question: Question #1. (Marks 20) Water and concentrated glucose solution are mixed to make diluted glucose syrup. The water feed contains pure water and its rate

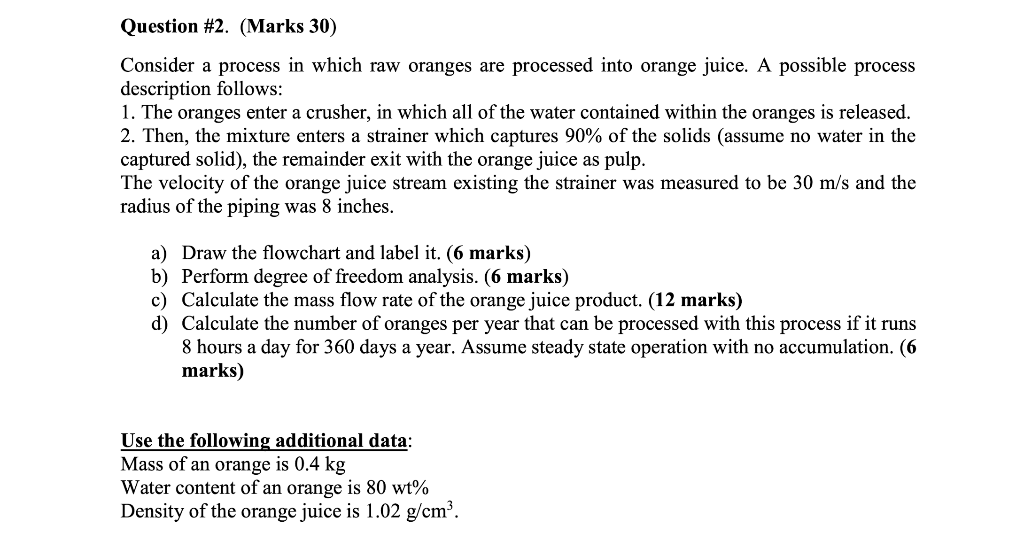
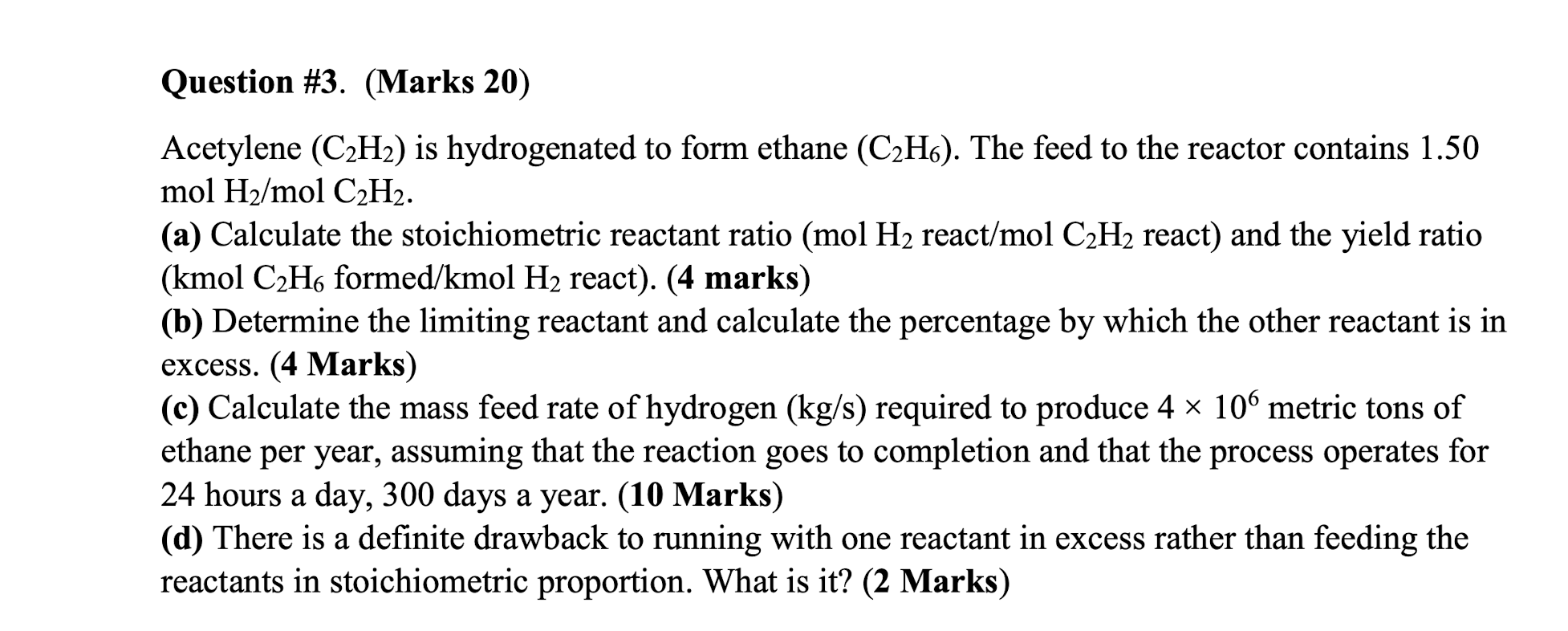
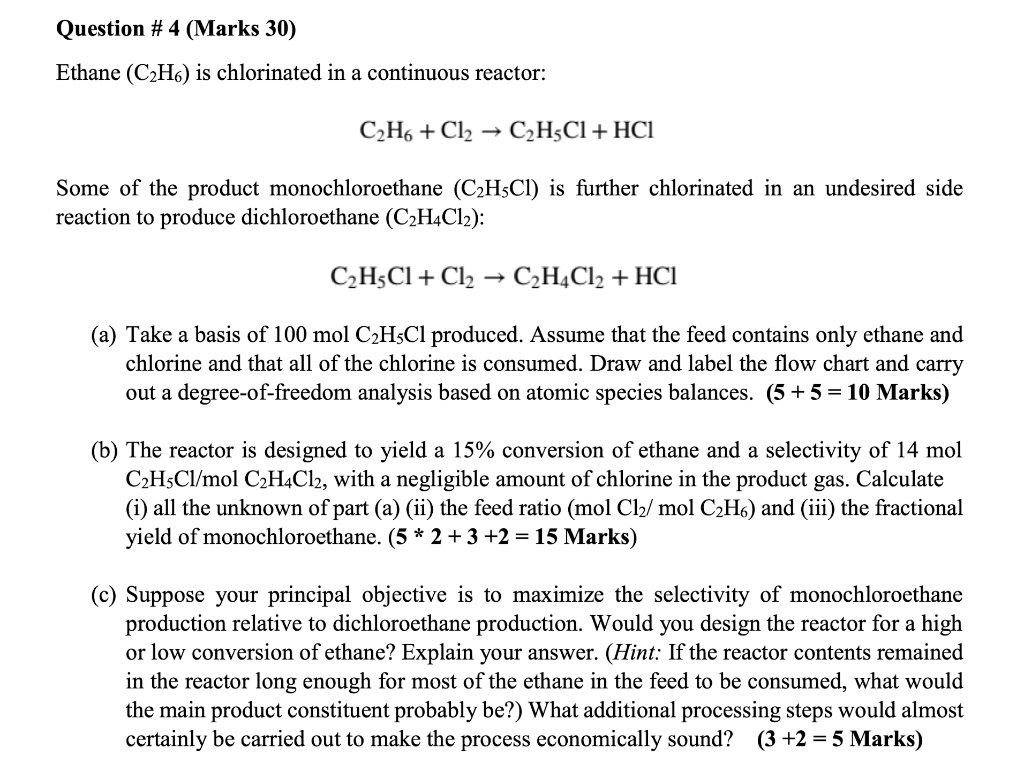
Question #1. (Marks 20) Water and concentrated glucose solution are mixed to make diluted glucose syrup. The water feed contains pure water and its rate is 500 kg/h while the concentrated glucose solution feed contains water and glucose at a mass ratio of 1:0.1. The product stream contains 5 wt% glucose and the rest is water. (a) Draw the flowchart and label it. (6 marks) (b) Perform degree of freedom analysis. (5 marks) (c) Calculate the flow rate of the concentrated glucose solution feed and the product stream. (9 marks) Question #2. (Marks 30) Consider a process in which raw oranges are processed into orange juice. A possible process description follows: 1. The oranges enter a crusher, in which of the water contained within the oranges is released. 2. Then, the mixture enters a strainer which captures 90% of the solids (assume no water in the captured solid), the remainder exit with the orange juice as pulp. The velocity of the orange juice stream existing the strainer was measured to be 30 m/s and the radius of the piping was 8 inches. a) Draw the flowchart and label it. (6 marks) b) Perform degree of freedom analysis. (6 marks) c) Calculate the mass flow rate of the orange juice product. (12 marks) d) Calculate the number of oranges per year that can be processed with this process if it runs 8 hours a day for 360 days a year. Assume steady state operation with no accumulation. (6 marks) Use the following additional data: Mass of an orange is 0.4 kg Water content of an orange is 80 wt% Density of the orange juice is 1.02 g/cm?. Question #3. (Marks 20) Acetylene (C2H2) is hydrogenated to form ethane (C2H6). The feed to the reactor contains 1.50 mol H2/mol C2H2. (a) Calculate the stoichiometric reactant ratio (mol H2 react/mol C2H2 react) and the yield ratio (kmol C2Ho formed/kmol H2 react). (4 marks) (b) Determine the limiting reactant and calculate the percentage by which the other reactant is in excess. (4 Marks) (c) Calculate the mass feed rate of hydrogen (kg/s) required to produce 4 x 106 metric tons of ethane per year, assuming that the reaction goes to completion and that the process operates for 24 hours a day, 300 days a year. (10 Marks) (d) There is a definite drawback to running with one reactant in excess rather than feeding the reactants in stoichiometric proportion. What is it? (2 Marks) a Question #4 (Marks 30) Ethane (C2H6) is chlorinated in a continuous reactor: C2H6 + Cl2 -> C2H3Cl + HCI Some of the product monochloroethane (C2H5Cl) is further chlorinated in an undesired side reaction to produce dichloroethane (C2H4Cl2): C2H3Cl + Cl2 C2H4Cl2 + HCI (a) Take a basis of 100 mol C2H5Cl produced. Assume that the feed contains only ethane and chlorine and that all of the chlorine is consumed. Draw and label the flow chart and carry out a degree-of-freedom analysis based on atomic species balances. (5 + 5 = 10 Marks) (b) The reactor is designed to yield a 15% conversion of ethane and a selectivity of 14 mol C2H5Cl/mol C2H4Cl2, with a negligible amount of chlorine in the product gas. Calculate (i) all the unknown of part (a) (ii) the feed ratio (mol Cl/mol C2H6) and (iii) the fractional yield of monochloroethane. (5 * 2 +3 +2 = 15 Marks) (c) Suppose your principal objective is to maximize the selectivity of monochloroethane production relative to dichloroethane production. Would you design the reactor for a high or low conversion of ethane? Explain your answer. (Hint: If the reactor contents remained in the reactor long enough for most of the ethane in the feed to be consumed, what would the main product constituent probably be?) What additional processing steps would almost certainly be carried out to make the process economically sound? (3 +2 = 5 Marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


