Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2: Pure Component Fugacity Calculation A. Determine the saturation fugacity of freon vapor at 475 R, using the van der Waals equation of state,
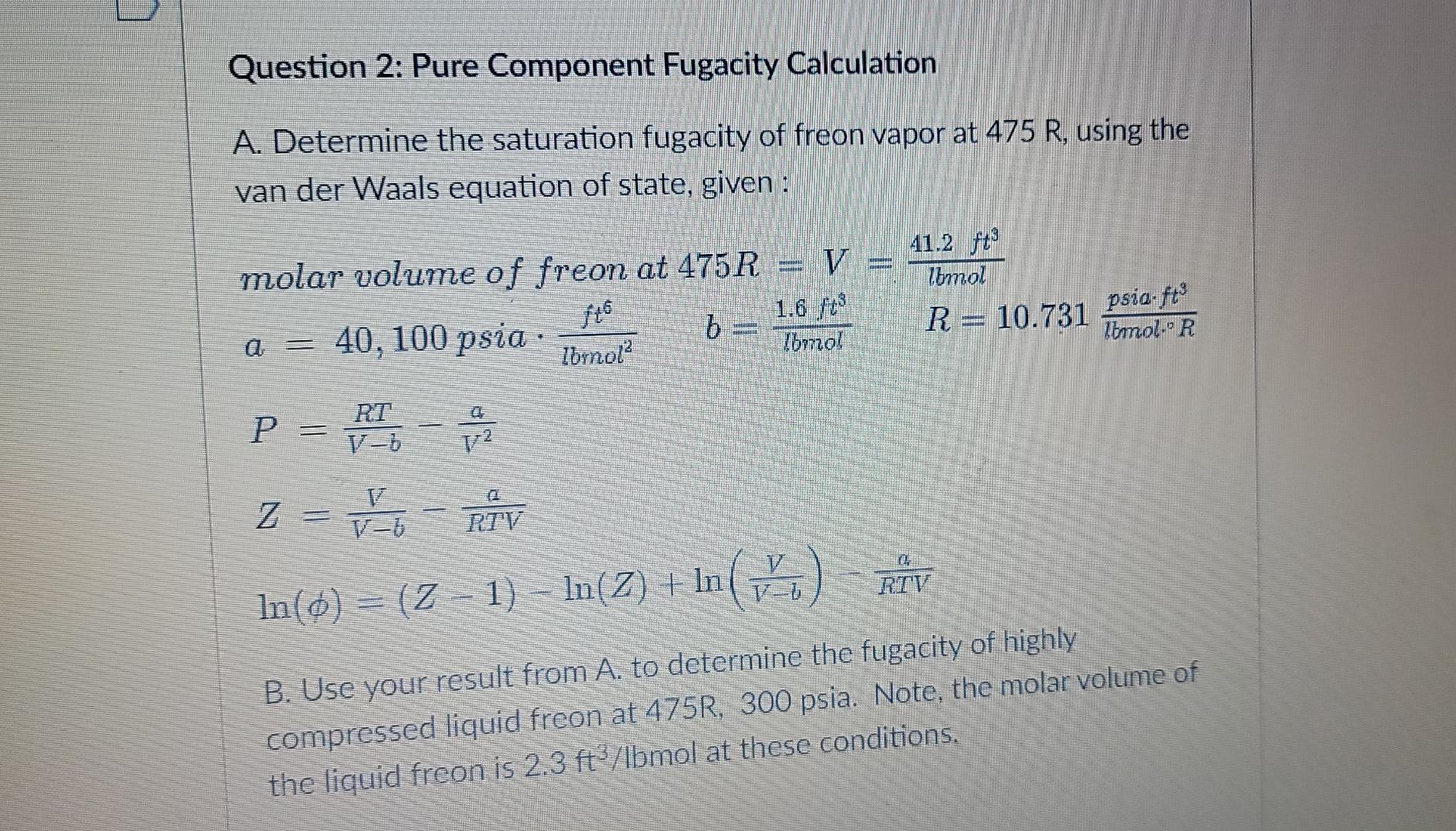
Question 2: Pure Component Fugacity Calculation A. Determine the saturation fugacity of freon vapor at 475 R, using the van der Waals equation of state, given: HOME molar volume of freon at 475R = V 1.6 ft 40, 100 psia b Ibrnol? Ibmol 41.2 f? Zomol R = 10.731 psia.ft foto lomolie R d RT P = R - Z = HT-RTV In(o)= 12 - 1) - In(2) + In (io) RIV Z- = VE B. Use your result from A. to determine the fugacity of highly compressed liquid freon at 475R, 300 psia. Note, the molar volume of the liquid freon is 2.3 ft3/Ibmol at these conditions
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


