Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Using the figure on the following page on how to achieve an octet for cations and anions, please draw a Lewis dot structure for

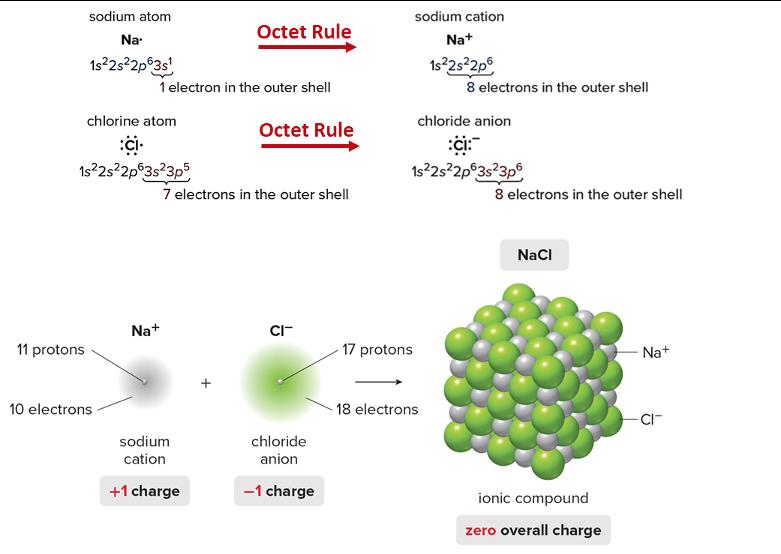
Using the figure on the following page on how to achieve an octet for cations and anions, please draw a Lewis dot structure for each of the following elements. Also, identify what net charge each element would adopt to achieve the valence electron configuration of a noble gas (achieve a stable octet). ***See Lithium below as an example. Li Lit where it has 1+ net charge a. Example: Li Lewis Structure: b. F Lewis structure: c. Mg Lewis structure: d. Cl Lewis Structure: e. K Lewis structure: f. S Lewis structure: g. Ca sodium atom Na- 1s2s2p63s 11 protons chlorine atom :CI 1s2s2p63s3p5 10 electrons 1 electron in the outer shell Na+ Octet Rule sodium cation +1 charge Octet Rule 7 electrons in the outer shell CI- chloride anion -1 charge sodium cation Na+ 1s2s2p6 17 protons chloride anion :ci: 1s2s2p63s3p6 8 electrons in the outer shell 18 electrons 8 electrons in the outer shell NaCl ionic compound zero overall charge Na+ -CI-
Step by Step Solution
★★★★★
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Solution z atomic number a Li15251 Lewis 23 5 b F H0 23 pps Lewis 29 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


