Give the products of reaction of propanoic acid with each of the following reagents. (a) SOCl2 (b)
Question:
Give the products of reaction of propanoic acid with each of the following reagents.
(a) SOCl2
(b) PBr3
(c) CH3CH2COBr 1 pyridine
(d) (CH3)2CHOH 1 HCl
(e) 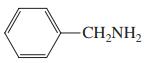
(f) Product of (e), heated strongly
(g) LiAlH4, then H+, H2O
(h) Br2, P
Transcribed Image Text:
HN HƆ-
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
CH CH OH Propanoic acid i CH CHC NHCH CHCOOH SOCI ii 3CHCHCOOH PBr Benzylamide iii CH3 CHCOOH ...View the full answer

Answered By

Manoj Upadhyay
I have teaching experience of 11 years.I have all abilities to solve the problem of chemistry.I always practice of short cuts for various problems.I also work as SME in various institution.I have worked as lecturer in many reputed college. I also worked as IIT OrJEE expert teacher.I believe that education is the best tool in order to get success.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the product of reaction of cyclopentanecarboxylic acid with each of the reagents in Problem 40. Data From problem 40 (a) SOCl2 (b) PBr3 (c) CH3CH2COBr 1 pyridine (d) (CH3)2CHOH 1 HCl (e) (f)...
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. i. j. HCI CH2CH3 1. CH3CH2MgBr 1. CH3CH2MgBr excesS CH,CH,COCH 2. H20 ot.cum 1. LiAIH4 NO 2. H20 catalytic Ht + CH...
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. HCI CH CH2CH CHCH2OH excess catalytic CCH CH3 NH2NH2 CH2CH3 NH2NH 1. NaBH4 0 HCI excess CH,CH,CH,COCH CHs 2. H3o 1. LIAIH...
-
A molecule with the molecular formula C11H12N2O2 (relative molecular mass = 204.23) crystallized to form monoclinic crystals with a=18.899 , b=5.7445 , and c=9.309 , with =101.776. The crystal...
-
Consider the following simple offshoring model of the type described in Section 11.2. The United States and Mexico both produce radios, using skilled and unskilled labor. Each radio requires three...
-
Suppose we are given two ordered dictionaries S and T, each with n items, and that S and T are implemented by means of array-based ordered sequences. Describe an O(log 2 n)-time algorithm for finding...
-
(Off-Balance-Sheet Financing) Brad Pitt Corporation is interested in building its own soda can manufacturing plant adjacent to its existing plant in Partyville, Kansas. The objective would be to...
-
Brightstone Tire and Rubber Company has capacity to produce 170,000 tires. Brightstone presently produces and sells 130,000 tires for the North American market at a price of $175 per tire....
-
If you invest $5,000, what is your rate of return if you will receive the following cash flows at the end of these years: Yr. 1. 2,000; Yr. 2. 2,000; Yr. 3. 2,000; Yr.4 4,000?
-
Matusek Corporation has been experiencing a higher than expected number of warranty claims in the current year, due mainly to less than ideal product design. For this reason, the warranty expense...
-
(a) Write a mechanism for the esterification of propanoic acid with 18 O-labeled ethanol. Show clearly the fate of the 18 O label. (b) Acid-catalyzed hydrolysis of an unlabeled ester with 18...
-
When methyl ketones are treated with a halogen in the presence of base, the three hydrogen atoms on the methyl carbon are replaced to give a CX 3 -substituted ketone. This product is not stable under...
-
Calculate the rms value of the waveform shown infigure. v(1) (V) + 8 10 12 4 14 t(s)
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
In a fixed time and budget project, the customer wants the development of a core component to be based on agile practices, as the final scope of the requirement has not yet been fully developed. The...
-
1. Ace Pizzeria, a manufacturer of frozen pizzas, computes its predetermined overhead rate annually based on machine hours. At the beginning of the year, the company estimated that 165,000 machine...
-
From a group of 13 boys and 9 girls, a committee of 5 students is chosen at random. a. The probability that all 5 members on the committee will be girls is (Type an integer or a simplified fraction.)...
-
On December 1, 2020, Cream Ale Ltd. receives $1,800 in advance for an agreement to brew beer during the months of December, January, and February. What is the revenue recognized under accrual...
-
Suppose that every monitor has a separate mutual exclusion lock, so that different threads can run in different monitors concurrently, and that we want to release exclusion on both inner and outer...
-
Extend Algorithms 3.4 and 3.5 to include as output the first and second derivatives of the spline at the nodes.
-
Identify the most acidic hydrogen in each of these compounds: a) HOCCH,CH,SOH 0 CO H 0 I e) CHCCHCOCHCH3 0 b) CH-CH,CH,C=N d) 0 CHOH f) HNCHCOH
-
Show the products of these acid-base reactions and predict whether the equilibria favor the reactants or the products: a) CHCCHCCHCH + OCHCH b) CHCHNO + CHO: CH3 (c) CH3COCH, + CHCH- 10 1:Z: LL CH,...
-
Which compound is behaving as the Lewis acid and which as the Lewis base in this reaction? AICI3 T CHCHCHCH3 + AICI CHCHCHCH3
-
George Johnson is preparing a valuation of Logistic Solutions, Inc. George has decided to use a two-stage DDM model and the following estimates. The Dividend per share is $1.75 for the current year...
-
Use a calculator to evaluate the expression. (Round your answer to four decimal places.) 32.6
-
52: What is implied by a bank credit that is known as a 7 percent rebate? 52: What is implied by a bank credit that is known as a 7 percent rebate

Study smarter with the SolutionInn App


