What are the products (and their ratios) of periodic acid cleavage of each of the following substances:
Question:
What are the products (and their ratios) of periodic acid cleavage of each of the following substances:
(a) 1,3-dihydroxyacetone;
(b) rhamnose (Problem 34);
(c) glucitol.
Problem 34
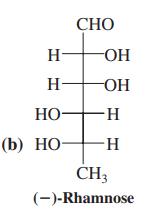
Transcribed Image Text:
СНО H- OH H OH НО H- (b) НО- -H- CH3 (-)-Rhamnosе
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
a b c HO 13 ...View the full answer

Answered By

Anurag Verma
I am a master in MSc chemistry with specialization in organic chemistry. I qualify national labvel exam of India like CSIR-JRF and GATE with good rank. I also passed various IIT interview of India for the admission in PhD. I also part time teacher and teach online and offline to a student of chemistry. I can solve any problem of organic chemistry also solve paper and teach spectroscopy, organomatellic chemistry, Inorganic chemistry also.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Predict the products formed by periodic acid cleavage of the following diols. (a) CH3CH(OH)CH(OH)CH3 (b) (c) (d) CHAOH OH OH Ph-CCH OH)CH,CH CH HO HO
-
What are the products of the following reaction? (a) C 6 H 5 I + CH 3 OH (b) C 6 H 5 OH + CH 3 I (c) C 6 H 5 I + CH 3 I (d) CH;OCH, HL A, HI. A, ?
-
What are the products of reaction of (E)-3-methyl-3-hexene with each of the reagents in Problem 46? (a) H 2 , PtO 2 , CH 3 CH 2 OH (b) D 2 , Pd C, CH 3 CH 2 OH (c) BH 3 , THF then NaOH + H 2 O 2 (d)...
-
ABC Company produces and sells I product. Once the products are produced, they are sold, and there is no work-in- process, no any inventory in stock. Company uses standard costing method in its...
-
Use the data in WAGEPRC.RAW for this exercise. Problem 11.5 gave estimates of a finite distributed lag model of gprice on gwage, where 12 lags of gwage are used. (i) Estimate a simple geometric DL...
-
What voltage must be applied to an x-ray tube for it to emit x-rays with a maximum frequency of 2 10 19 Hz?
-
1.1 Ordered Field Axioms 2. (a) (-3,7). (b) (-3,5). (c) (-1,-1/2) U (1,00). (d) (-2,1).
-
Assume that Chapman Company acquired Abernethys common stock for $500,000 in cash. Assume that the equipment and long-term liabilities had fair values of $220,000 and $120,000, respectively, on the...
-
You are using the Black-Scholes model and need the value of N(d1). If dy is 0.85, N(d) is__ O 0.8023 O 0.15 O 0.1977 O 0.2119
-
On January 15, 2015, Sports World sold 1,000 Ace-5 fishing reels to Angler's Warehouse. Immediately prior to this sale, Sports World perpetual inventory records for Ace-5 reels included the following...
-
Ketoses show positive Fehlings and Tollenss tests not only by oxidation to -dicarbonyl compounds, but through a second process: Ketoses isomerize to aldoses in the presence of base. The aldose then...
-
Write the expected products of the reaction of each of the following sugars with (i) Br 2 , H 2 O; (ii) HNO 3 , H 2 O, 60C; (iii) NaBH 4 , CH 3 OH; and (iv) excess C 6 H 5 NHNH 2 , CH 3 CH 2 OH, ....
-
Explain the limitations of the trial balance.
-
Identify the following; MethodBodyReturn statementReturn typeParameter Look at this example we saw in our Methods lesson: public double findTheArea (double length, double w idth) { double area =...
-
Machine-hours required to support estimated production Fixed manufacturing overhead cost Variable manufacturing overhead cost per machine-hour Required: 1. Compute the plantwide predetermined...
-
Assume now that a new firm (firm N) discovers and patents a more efficient technology, summarized by thetotal cost function C = 10q. The new technology can be used only by the new firm, which enters...
-
1. How has Dell used virtual integration to become an industry leader? Dell has used virtual integration to become an industry leader by leveraging its global suppliers to reduce costs and provide...
-
3) Consider the asset pricing model with uncertainty in the slide. We derived the asset prices as Pb = Ps = - [nu' (y+Yn + e) + (1 )u' (y + y + e)] u'(e1) [nu' (y +n + ) + (1 )u' (y + y + e2)] u'(e1)...
-
Write a program that converts dates from numerical month-day format to alphabetic month-day format. For example, input of 1/31 or 01/31 would produce January 31 as output. The dialogue with the user...
-
Privitera and Freeman (2012) constructed a scale to measure or estimate the daily fat intake of participants; the scale was called the estimated daily intake scale for fat (EDIS-F). To validate the...
-
What aromatic products would you obtain from the KMnO4 oxidation of the followingsubstances? (a) O2N. (b) C(CH3)3 CH(CH3)2 "
-
Refer to Table 5.3 for quantitative idea of the stability of a benzyl radical. How much more stable (in kJ/mol) is the benzyl radical than a primary alkyl radical? How does a benzyl radical compare...
-
Styrene, the simplest alkenylbenzene, is prepared commercially for use in plastics manufacture by catalytic dehydrogenation of ethylbenzene. How might you prepare styrene from benzene using reactions...
-
Fixed cost per unit is $7 when 25,000 units are produced and $5 when 35,000 units are produced. What is the total fixed cost when 30,000 units are produced? Group of answer choices $150,000....
-
During the year 2021, William has a job as an accountant, he earns a salary of $100,000. He has done some cleaning services work on his own (self-employed), where he earned a net income of $50,000....
-
Which of the following requirements to claim Earned Income Tax Credit is TRUE? The credit can be claimed under any filing status. The taxpayer must have a valid SSN for employment in the U.S., issued...
IES 2012 Mechanical Engineering Objective Solved Paper II 1st Edition - ISBN: 9381069875 - Free Book

Study smarter with the SolutionInn App


