Which common analytical method will most clearly and rapidly distinguish A from B? (a) IR spectroscopy; (b)
Question:
Which common analytical method will most clearly and rapidly distinguish A from B?
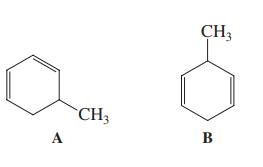
(a) IR spectroscopy;
(b) UV spectroscopy;
(c) combustion analysis;
(d) visible spectroscopy
Transcribed Image Text:
CH3 CH3 A B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
The correct answer is B UV ultraviolet spectroscopy Com...View the full answer

Answered By

Vivek Kumar
Hi. I am Postgraduate in Chemistry (2012) with First class degree.
Expert in time bound exam assignment and quizzes. Did for many students
Seasoned online chemistry Tutor with 4 years of experience in tutoring across the globe.
Students loves my lessons and i get lots of appreciations and complements. I teach every part of chemistry ranging from Physical chemistry to Medicinal chemistry.
If you ask me speciality, i would say Organic chemistry, Spectroscopy (MS, NMR IR and UV etc) and Physical chemistry.
I am also tutoring maths.
I believe teaching in an art. Not every student is same and so the subject. Therefore it is the responsibility of Tutor to use that art to help students and make the subject interesting.
Thank You. Keep Smiling and keep learning
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Explain how IR spectroscopy could be used to distinguish between thesecompounds: and b) and NH2 d d) and and e) ) and
-
Explain how UV spectroscopy could be used to distinguish between thesecompounds. and a) b) and c) and HO and d)
-
(a) Infrared spectroscopy provides an easy method for deciding whether the product obtained from the addition of a Grignard reagent to an α,β-unsaturated ketone is the...
-
Operating Rules. Indicate whether the following statements are true or false. If the statement is false, explain why. a. Since most corporations make distributions only when they are profitable and...
-
Which one(s) of the methodologies described in this chapter do you feel would be the most difficult to manage? Explain. Which do you think is the most successful? Why?
-
Clark Company's current year income statement, comparative balance sheets, and additional information follow. For the year. (1) all sales are credit sales. (2) all credits to Accounts Receivable...
-
The authority of managers to exercise discipline in relation to others in the organisation is underpinned by a general predilection of people to obey commands from those holding higher rank in the...
-
CVP computations Patel Manufacturing sold 200,000 units of its product for $30 per unit In 2008. Variable cost per unit is $25 and total fixed costs are $800,000. 1. Calculate la) contribution margin...
-
In its IPO, 1 million company shares were issued at a price of $28 per share. The underwriter's fee for the offering was 10% of gross proceeds and the issuer incurred direct out-of-pocket costs of...
-
Required a). Consider using a simple moving average model. Experiment with models using five weeks and three weeks past data. (Round your answers to 2 decimal places.)rn b). Evaluate the forecasts...
-
When cyclopentadiene is treated with tetracyanoethene, a new product results. Its most likely structure is CN CN CN () - CN CN (b) N N CN CN CN H,C-C-CN () H2C-C-CN (d) CN NC CN CN
-
Name each of the following compounds by using the IUPAC system and, if possible, a reasonable common alternative. (The order of functional group precedence is COOH >. CHO > OH> NH 2 .)
-
At the end of 2010, Rowet Company reported a deferred tax liability of $6,120 based on an income tax rate of 30%. On June 1, 2011 Congress changed the income tax rate to 35%. Required 1. Calculate...
-
Which of the five strategies for adapting products and promotion for global markets does Monster Employ? 15-16. Which factors in the global marketing environment have challenged Monster's global...
-
Analysis of Current International Economic Environment in Switzerland 1. Develop a lead sentence for this section that introduces the key subsections 2. Economic Environment describe Switzerland...
-
1.1 Indonesia is it potential as a market for Apple? 2.1 Examination of Apple's entry strategy into the international market? 2.2 Evaluation of the entry mode(s) employed by Apple and their...
-
Dynamic, a global media agency, has recently taken over MediaHype, a local agency in Melbourne, to expand its Australian operations. Jeff Tan, a Chinese national, has been appointed to head the new...
-
Linear optimization models play a crucial role in improving supply chain management efficiency, both in physical and abstract network problems. Three ways they can be applied are through optimizing...
-
Howard Cooper, the president of Glacier Computer Services, needs your help. He wonders about the potential effects on the firms net income if he changes the service rate that the firm charges its...
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
Treat carbon monoxide as a perfect gas and apply equilibrium statistical thermodynamics to the study of its properties, as specified below, in the temperature range 100-1000 K at 1 bar. V = 2169.8...
-
The exchange of deuterium between acid and water is an important type of equilibrium, and we can examine it using spectroscopic data on the molecules. Calculate the equilibrium constant at (a) 298 K...
-
Suppose that an intermolecular potential has a hard-sphere core of radius 'I and a shallow attractive well of uniform depth E out to a distance '2' Show, by using eqn 17.42 and the condition E kT,...
-
For esch of the following Independent tranactiona, determine the minimum amount of net income or loas for tox purposes snd the tsxpsyer to which it applies. 1 An individual purchases a $ 1 0 , 0 0 0...
-
Suppose a bond has a modified duration of 4. By approximately how much will the bonds value change if interest rates: a. Increase by 50 basis points b. Decrease by 150 basis points c. Increase by 10...
-
Difference between Operating Leverage and Financial Leverage

Study smarter with the SolutionInn App


