Question: The following mixture is introduced into a distillation column as saturated liquid at 1.72MPa. Calculate the bubble-point temperature using the K-values of Figure. Compound kmol/h
The following mixture is introduced into a distillation column as saturated liquid at 1.72MPa. Calculate the bubble-point temperature using the K-values of Figure.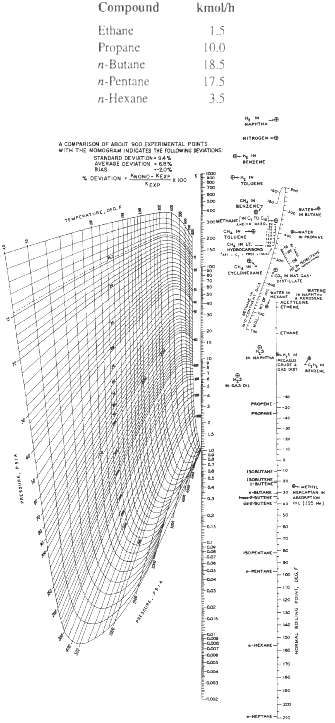
Compound kmol/h Ethane 1.5 Propane 10.0 n-Butane 18.5 n-Pentane 17.5 - 35 HITROGEHO A COMPARISON DF ARCIT soD FPLRIMENTAL PONTS WITH THE MOMGRAM INDICATES HE FouwNE EVATIONS: STANDAND DEVUT ON - 34% AVERADE CEVIATION % Ries * DEVIATON UN-KE KEKP BATE HLTA CH IH LT. ETELIHEANE BISTATE ACESYLENE PROP kas Facod 4003
Step by Step Solution
3.22 Rating (169 Votes )
There are 3 Steps involved in it
Iterate on temperature until the bubblepoin... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (120).docx
120 KBs Word File


