What ester an what Grignard reagent might you start with to prepare the following alcohols? (b) ,
Question:
What ester an what Grignard reagent might you start with to prepare the following alcohols?
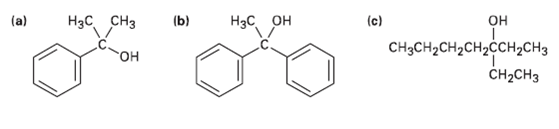
Transcribed Image Text:
(b) Нас, сНз Hас он (c) он сHзCH-сH2сH2ссн,сHз CH-CHз (a) он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
Strategy Remember that Grignard reagents can only be used with esters to form a terti...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What carbonyl compounds might you start with to prepare the following compounds by Grignard reaction? List all possibilities. (a) 2-Methyl-2-propanol (b) 1-Ethylcyclohexanol (c) 3-Phenyl-3-pentanol...
-
What alkynes would you start with to prepare the followingketones? (a) (b) CHCHH2H CH3CH2CH2CH3
-
What alkyne would you start with to prepare each of the following compounds by a hydroboration/oxidationreaction? (b) (a) CH CH-CCHCH3 -CH2CH CH
-
In a recent year, the total scores for a certain standardized test were normally distributed, with a mean of 500 and a standard deviation of 10.4. Answer parts (a)-(d) below. (a) Find the probability...
-
Discuss the advantages and disadvantages of using video résumés and other creative but unconventional job application strategies.
-
Other than financial assistance, how might industrialized countries help developing countries to control ozone depletion?
-
RESIDUAL DIVIDEND MODEL Welch Company is considering three independent projects, each of which requires a $5 million investment. The estimated internal rate of return (IRR) and cost of capital for...
-
It is fairly common for an industrial cluster to break up and for production to move to locations with lower wages when the technology of the industry is no longer rapidly improvingwhen it is no...
-
A Corp makes distribution of an asset to B Corp FMV $100,000; Basis $300,000. Does A Corp recognize a loss when it makes the distribution?
-
You are BA for an organization XYZ who have products for BPM (Business Process Management) and Document Management System to enable business process improvement for customer AER Bank. AER Bank wants...
-
Show the products you would obtain by reduction of the following esters withLiAlH4: (b) (a) CHH2CH2
-
How would you convert N-ethylbenzamide to each of the following products? (a) Benzoic acid (b) Benzyl alcohol (c) C 6 H 5 CH 2 NHCH 2 CH 3
-
Do the following. (a) Graph y = f(x). (b) Use the graph of y = f(x) to sketch a graph of the equation y = |f(x)|. (c) Determine the x-intercept for the graph of y = |(x)|. y = 2 - 4x
-
The figure shows a turbine-driven pump that provides water, at high pressure, to a tank located 25-m higher than the pump. Steady-state operating data for the turbine and the pump are labelled on the...
-
Step 1 Step 2 1. Sketch what step 4 and then step 5 would look like. Step 4 Step S 2. How many black triangles are in each step? Step 1 black A = | Step 2 = 4 black A's step 3 = 13 black D's 3. What...
-
The pressure cooker pictured here consists of a light pressure vessel with a heavy lid of weight W. When the lid is secured, the vessel is filled with a hot pressurized gas of pressure p. After some...
-
5) A large group of students took a test in Finite Math where the grades had a mean of 72 and a standard deviation of 4. Assume that the distribution of these grades is approximated by a normal...
-
Q9 (5 points) According to Dr. Henry Mintzberg, a noted management scholar from McGill University in Montreal, PQ, "business organizations perform only two activities of consequence." What are these...
-
Victor Ortega is the owner of a very successful small company that operates a carry-out pizza parlor. He also prepares his own financial statements. His task today is the completion of his 2011...
-
The unadjusted trial balance of Secretarial Services is as follows: SECRETARIAL SERVICES Unadjusted Trial Balance as at 31 December 2017 Account Debit Credit Cash at bank Office supplies Prepaid...
-
Sketch the bonding and antibonding molecular orbitals that result from linear combinations of the 2p z atomic orbitals in a homonuclear diatomic molecule. (The 2p z orbitals are those whose lobes are...
-
Predict the relative intensities of the three peaks in the mass spectrum of dichloromethane at m/z = 84, 86, and 88.
-
From the molecular masses and the relative intensities of their M and M + 1 peaks, suggest molecular formulas for the following compounds. M (m/z = 82; 37%), M + 1 (2.5%); contains C and H.
-
Suggest a structure for each of the ions corresponding to the following peaks in the EI mass spectrum of ethyl bromide, and give a mechanism for the formation of each ion. (The numbers in parentheses...
-
explain in excel please For a particular product the price per unit is $6. Calculate Revenue if sales in current period is 200 units. Conduct a data analysis, on revenue by changing the number of...
-
Hall Company sells merchandise with a one-year warranty. In the current year, sales consist of 35,000 units. It is estimated that warranty repairs will average $10 per unit sold and 30% of the...
-
Q 4- Crane Corporation, an amusement park, is considering a capital investment in a new exhibit. The exhibit would cost $ 167,270 and have an estimated useful life of 7 years. It can be sold for $...

Study smarter with the SolutionInn App


