Prove that the normalization constant of the 1s radial wave function of the hydrogen atom is (a
Question:
Prove that the normalization constant of the 1s radial wave function of the hydrogen atom is (πaB3)–1/2, as given in Equations 41.7. A useful definite integral is
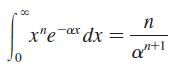
Transcribed Image Text:
00 n x"e ax dx = xp,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Solve The normalization conditio...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
Prove that the normalization constant of the 2p radial wave function of the hydrogen atom is (24a B 3 ) 1/2 , as shown in Equations 41.7. 1 R1,(r) = -rlag 1 ap -r/2ag R2,(r) 1 3 2ag 1 r R(r) -r/2ag e...
-
The wave function for a hydrogen atom in the 2s state is(a) Verify that this function is normalized.(b) In the Bohr model, the distance between the electron and the nucleus in the n = 2 state is...
-
Prove that the normalization factor given by (12.2.12) ensures that (12.2.19) issatisfied. M-1 Ew?(n) (12.2.12) W(f)df = 1 J-1/2 (12.2.19)
-
In your own words, define or explain the terms or symbols (a) (b) [ ]; (c) Spectator ion; (d) Weak acid.
-
Reconsider Prob. 1-102. Using EES (or other) software, investigate the effect of the number of people carried in the balloon on acceleration. Plot the acceleration against the number of people, and...
-
An auditor is using sampling while testing internal controls. To determine whether the results of the tests are acceptable, the auditor compares the computed upper exception rate (CUER) with: Group...
-
What are the various components of a marketing strategy?
-
A survey showed that a majority of Americans plan on doing their holiday shopping online because they dont want to spend money on gas driving from store to store (SOASTA website, October 24, 2012)....
-
I need help filling out this excel document in full. Please help me fill every green slot, the bottom equation, and summary sentences on the bottom right. Thank you!
-
List seven programming languages that are procedural and two that are nonprocedural. Which group is easier to learn and use? Explain your answer.
-
Prove that the radial probability density peaks at r = a B for the 1s state of hydrogen.
-
For an electron in the 1s state of hydrogen, what is the probability of being in a spherical shell of thickness 0.010a B at distance (a) 1/2 a B (b) a B (c) 2a B from the proton?
-
Sketch ABC. Find the length and the slope of each side. Then find the coordinates of each midpoint. Is ABC a right triangle? Is it isosceles? Explain. (Assume all variables are positive, p q, and m ...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
The accounts in the ledger of Angel Co. as of December 31, 20Y7, are listed in alphabetical order as follows. All accounts have normal balances. The balance of the cash account has been intentionally...
-
What are technical skills At what level are they most important and why?
-
A 20 g ball of clay traveling east at 2.0 m/s collides with a 30 g ball of clay traveling 30 south of west at 1.0 m/s. What are the speed and direction of the resulting 50 g blob of clay?
-
A 20 g ball of clay traveling east at 2.0 m/s collides with a 30 g ball of clay traveling 30 south of west at 1.0 m/s. What are the speed and direction of the resulting 50 g blob of clay?
-
FIGURE P11.71 shows a collision between three balls of clay. The three hit simultaneously and stick together. What are the speed and direction of the resulting blob of clay? 40 g JASO 45 4.0 m/s 3.0...
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


