Some of the most important metals we use are found in the transition element section of the
Question:
Some of the most important metals we use are found in the transition element section of the Periodic Table. One of these elements is copper. Sodium, a Group I metal, has very different properties from those of copper. Complete the table below to show their differences.
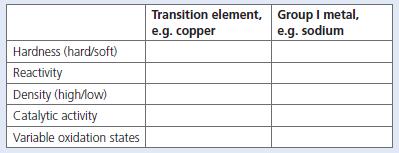
Transition element, Group I metal, e.g. sodium e.g. copper Hardness (hard/soft) Reactivity Density (high/low) Catalytic activity Variable oxidation states
Step by Step Answer:
Transition element copper soft malleable and ductile Copper is low on reactivi...View the full answer



Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Sciences questions
-
Some of the most important organic compounds in biochemistry are the _-amino acids, represented by the general formula shown.
-
The following are some of the most important trigonometric identities. Check them at a. = 0, b. = /4, c. = /2, d. = . cos (/2) = 1 + cos()/2. Only check at points a, c, and d.
-
The following are some of the most important trigonometric identities. Check them at a. = 0, b. = /4, c. = /2, d. = . sin2() + cos2() = l.
-
Suppose treacle is an array of 10 floats. Declare a pointer that points to the first element of treacle and use the pointer to display the first and last elements of the array.
-
For each of the following, write the complete chemical equation for the acidbase reaction that occurs. Describe each using Brnsted language (if appropriate) and then using Lewis language (show...
-
This problem continues the Draper Consulting, Inc., situation from Problem 23-39 of Chapter 23. Draper Consulting reported 2013 sales of $3,750,000 and operating income of $210,000. Average total...
-
Verify that each of the terms in the sum-of-squares function (see Equation 9.9 on page 208) Sb y0 y ( y0 Xb ( b0 X0 y b0 X0 Xb is (1 1), justifying writing Sb y0 y ( 2y0 Xb b0 X0 Xb
-
Peterman Co. was organized on July 1, 2012. Quarterly financial statements are prepared. The unadjusted and adjusted trial balances as of September 30 are shown below. Instructions (a) Journalize the...
-
20. The part X10 is used in the production of one of the products manufactured by Jemo Company. Each year, a total of 50,000 units of this part are produced and used. The costs of producing the part...
-
A piece of cork having a specific weight of 2.36 kN/m 3 is shaped as shown in Fig. 5.32. (a) To what depth will it sink in turpentine (sg = 0.87) if placed in the orientation shown? (b) Is it stable...
-
Use the following list of metals to answer the questions a to i: iron, calcium, potassium, gold, aluminium, magnesium, sodium, zinc, platinum, titanium. a. Which of the metals is found native? b....
-
a. Consider the chemical properties and physical properties of the halogens chlorine, bromine and iodine. Using these properties, predict the following about the other two halogens, fluorine and...
-
Refer to the Fourth Street Floral data in E6-51B. Use Microsoft Excel to run a regression analysis, then do the following calculations: Requirements: 1. Determine the firms cost equation (use the...
-
If you are a Super Coffee company and want to partner with Influencers on Instagram. How to find them? Influencer suggestions? How much to pay them? Who are they? Your budget is $250,000 So...
-
As the Customer Support Manager in the Fast-Moving Consumer Good (FMCG) sector with the ABC Corporation you are expected to address major grievances of customers from our product line and special...
-
An agent for positive change can be defined as someone who has the capability to influence and motivate those around them to accomplish whatever shared task needed to achieve a common goal, these...
-
Identify stress-reduction techniques used in organizations (e.g., wellness programs) Evaluate the impact of personal and work-related stress on performance Explain the relationship between...
-
Company: Viventium Explain which metrics you recommend tracking and why. For example, "these are the 3 to 5 metrics I've chosen; I'm tracking this because it does X, thereby bringing me closer to...
-
Karen Austin Inc. has issued three types of debt on January 1, 2020, the start of the companys fiscal year. a. $10 million, 10-year, 15% unsecured bonds, interest payable quarterly. Bonds were priced...
-
The purpose of this case is to come up with a contingency plan[s] in order to sustain the program Move With Me, a program that serves thousands of community members throughout Lower Manhattan. The...
-
When 2.25 mg of anthracene, CI4HlO(s), was burned in a bomb calorimeter the temperature raised by 1.35 K. Calculate the calorimeter constant. By how much will the temperature rise when 135 mg of...
-
Calculate the standard enthalpy of solution of AgBr(s) in water from the enthalpies of formation of the solid and the aqueous ions.
-
Given that the standard enthalpy of combustion of graphite is -393.51 kJ mol-1 and that of diamond is -395.41 kJ mol-1, calculate the enthalpy of the graphite-to-diamond transition.
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


