The feed to a pair of gas permeators connected with retentate in series is (40 mathrm{~mol} %)
Question:
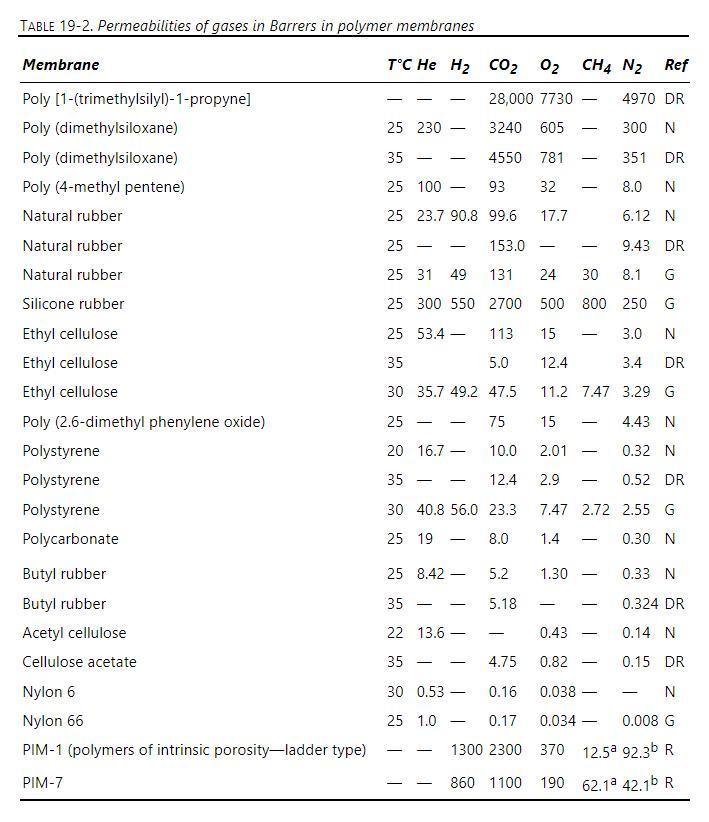
The feed to a pair of gas permeators connected with retentate in series is \(40 \mathrm{~mol} \%\) carbon dioxide and 60 \(\mathrm{mol} \%\) nitrogen. The perfectly mixed membrane separators are equipped with silicone rubber membranes with effective membrane thicknesses of \(0.9 \mu \mathrm{m}\). In both units, the pressure on the permeate side is \(1.07 \mathrm{bar}\) and the retentate pressure is \(5.3 \mathrm{bar}\). The membrane units operate at \(25^{\circ} \mathrm{C}\). Feed rate to the first unit is \(120.0 \mathrm{kmol} / \mathrm{h}\). Assume an ideal gas. Data are in Table 19-2.
a. Can a single unit produce the desired retentate with \(\mathrm{y}_{\mathrm{r}, \mathrm{CO} 2}=0.16\) ?
b. Whether or not a single membrane will work, design a system with two units in series because a higher flow rate of desired retentate can be obtained. We want the retentate from the second membrane unit to be \(\mathrm{y}_{\mathrm{r} 2, \mathrm{CO} 2}=0.16\). Design the system (provide values of \(\theta_{1}\) and \(\theta_{2}\) and report resulting values of \(\mathrm{F}_{\mathrm{r} 1}, \mathrm{~F}_{\mathrm{r} 2}, \mathrm{y}_{\mathrm{p} 1}\), \(\mathrm{y}_{\mathrm{r} 1}, \mathrm{y}_{\mathrm{p} 2}\), and \(\mathrm{y}_{\mathrm{r} 2}\) ) to obtain the highest flow rate of retentate gas \(\left(\mathrm{F}_{\mathrm{r} 2}\right)\) as possible.
If the equations are set up in a spreadsheet, first find the value of \(\mathrm{y}_{\mathrm{p} 2}\) that corresponds to specified \(\mathrm{y}_{\mathrm{r} 2}\). A case study approach (pick a value of \(\mathrm{y}_{\mathrm{p} 1}\), calculate \(\mathrm{y}_{\mathrm{r} 1}=\mathrm{y}_{\mathrm{in}, 2}\) from the RT equation, calculate \(\theta_{1}\) and \(\theta_{2}\), then find \(\mathrm{F}_{\mathrm{r} 2}\) and repeat for different values of \(\mathrm{y}_{\mathrm{p} 1}\) ) works well.
\(\mathrm{y}_{\mathrm{p} 1}, \mathrm{y}_{\mathrm{r} 1}, \mathrm{y}_{\mathrm{p} 2}\), and \(\mathrm{y}_{\mathrm{r} 2}\) are mole fractions of the faster permeating gas.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





