Calculate (Delta S_{text {surr }}) for the following reactions at (25^{circ} mathrm{C}) and (1 mathrm{~atm}). a. C3H8(g)
Question:
Calculate \(\Delta S_{\text {surr }}\) for the following reactions at \(25^{\circ} \mathrm{C}\) and \(1 \mathrm{~atm}\).
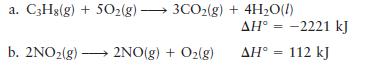
Transcribed Image Text:
a. C3H8(g) + 5O(g) 3CO2(g) + 4HO(l) b. 2NO2(g) 2NO(g) + O(g) AH = -2221 kJ AH = 112 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (8 reviews)
The change in entropy of the surroundings Delta Stextsurr for a reaction can be determined by lookin...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate DG and Kc for the following reactions at 25C: (a) (b) (c) (d) 2 + Br2() 21 (aq)2Br (aq) I2(s) 02(8) 4H (a) 4Fe (aq) 2H20(1) + 4Fe (aq) 2.A l (s) + 312(s) 2A13+(aq) + 6(aq)
-
Calculate ÎSsurr for the following reactions at 25oC and 1 atm. a. C3Hsg) 502(g)3CO2ig) 4H20(D -2221 kJ ' '-112 kJ b, 2N02(g)-2N0(g) + O2(g)
-
The equilibrium constant of the reaction CO + ½ O2 CO2 at 1000 K and 1 atm is KP1 Express the equilibrium constant of the following reactions at 1000 K in terms of KP1: (a CO10CO at 3 atm at 1...
-
In your hometown what system is used to price the publicly supplied water? Why was that pricing system chosen? Would you recommend an alternative?
-
Consider the Henon map described by Let a = 1.4 and b = 0.3, and use a computer to plot the first 10,000 points (xw yn) starting from the initial values x0 = 0, y0 =0. Choose the plot region as 1.5 -...
-
Obtain a linear approximation of the function f(h) = h valid near h = 16. Noting that f(h) 0, what is the value of h below which the linearized model loses its meaning?
-
Describe the transformation process. Apply the open systems model in Figure 12.1 to an organisation that you know.
-
Digital Access Inc. needs $400,000 in funds for a project. a. With a compensating balance requirement of 20 percent, how much will the firm need to borrow? b. Given your answer to part a and a stated...
-
Which company compares to Bloomberg Market's performance in the industry?
-
Given the following data: calculate \(\Delta G^{\circ}\) for the reaction \[6 \mathrm{C}(s)+3 \mathrm{H}_{2}(g) \longrightarrow \mathrm{C}_{6} \mathrm{H}_{6}(l)\] 2C6H6(l) +150(g) 12CO(g) + 6HO(l)...
-
Choose the compound with the greatest positional probability in each case. a. \(1 \mathrm{~mol}_{\text {of }} \mathrm{H}_{2}\) at STP or \(1 \mathrm{~mol}_{\text {of }} \mathrm{H}_{2}\) at...
-
Find the area of the region bounded above by y = e x , bounded below by y = x, and bounded on the sides by x = 0 and x = 1.
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
A gas-turbine power plant operates on the simple Brayton cycle between the pressure limits of 100 and 800 kPa. Air enters the compressor at 30C and leaves at 330C at a mass flow rate of 200 kg/s. The...
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
The rate law of the reaction 2 NO(g) + 2 H 2 (g) N 2 (g) + 2 H 2 O(g) is Rate = kr[NO] 2 [H 2 ], and the mechanism that has been proposed is (a) Which step in the mechanism is likely to be rate...
-
(a) Use a graphing calculator or graphing software to calculate the activation energy for the acid hydrolysis of sucrose to give glucose and fructose (see Exercise 7.9) from an Arrhenius plot of the...
-
The decomposition of A has the rate law Rate = k r [A] a . Show that for this reaction the ratio t 1/2 /t 3/4 , where t 1/2 is the half-life and t 3/4 is the time for the concentration of A to...
-
Kenneth lived in his home for the entire year except for when he rented his home (near a very nice ski resort) to a married couple for 14 days in December. The couple paid Kenneth $14,000 in rent for...
-
On December 31, 2021, Shack Store Inc had 143 million shares outstanding, which traded for $643.29 per share. On January 02, 2022, the CEO announced a 20-for-1 stock split. Every shareholder would...
-
o1s= secom o1s= secom

Study smarter with the SolutionInn App


