(a) Use a graphing calculator or graphing software to calculate the activation energy for the acid hydrolysis...
Question:
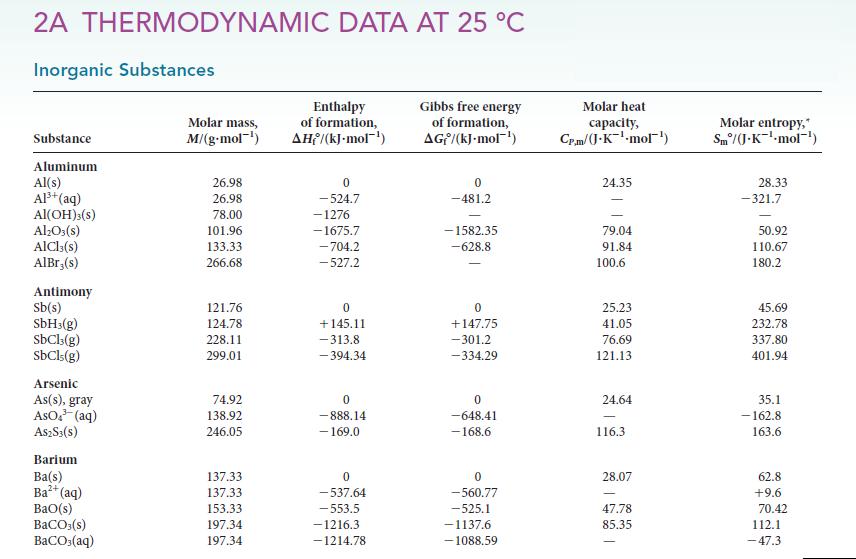
(a) Use a graphing calculator or graphing software to calculate the activation energy for the acid hydrolysis of sucrose to give glucose and fructose (see Exercise 7.9) from an Arrhenius plot of the data below.
(b) Calculate the rate constant at 37°C (human body temperature).
(c) From data in Appendix 2A, calculate the enthalpy change for this reaction, assuming that the solvation enthalpies of the sugars are negligible. Draw an energy profile for the overall process.![]()
Exercise 7.9
The hydrolysis of sucrose (C12H22O11) produces fructose and glucose: C12H22O11(aq) + H2O(l) → C6H12O6(glucose, aq) + C6H12O6 (fructose, aq). Two mechanisms are proposed for this reaction: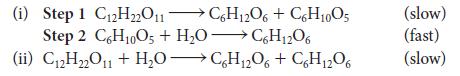
Under what conditions can these two mechanisms be distinguished by using kinetic data?
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





