Methanol is a high-octane fuel used in high-performance racing engines. Calculate G for the reaction given the
Question:
Methanol is a high-octane fuel used in high-performance racing engines. Calculate ΔG° for the reaction
![]()
given the following free energies of formation:
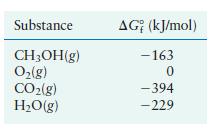
Transcribed Image Text:
2CHOH(g) + 30(g) 2CO(g) + 4HO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
We use AG EAG products AG reactants 2AG COg 4AG H...View the full answer

Answered By

Muhammad Rehan
Enjoy testing and can find bugs easily and help improve the product quality.
4.70+
10+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
The following data compare the standard enthalpies and free energies of formation of some crystalline ionic substances and aqueous solutions of the substances: (a) Write the formation reaction for...
-
In the Mond process for the purifi cation of nickel, carbon monoxide is reacted with heated nickel to produce Ni(CO)4, which is a gas and can therefore be separated from solid impurities: Ni(s) +...
-
Changing preferences can also affect changes in land use. In the United States, the proportion of the population in the 65-and-older age bracket is growing. What effects might this have on the...
-
Consider a projectile fired vertically in a constant gravitational field. For the same initial velocities, compare the times required for the projectile to reach its maximum height (a) For zero...
-
Consider the-chapter opening case. What are the advantages that Fieldf/x provides for the owners of professional baseball teams? What are the advantages that Fieldf/x provides for professional...
-
Describe the system they implemented, especially the hardware and software.
-
1. What should an employee do when he or she discovers that there is an error in a projection? Why do you suggest that action? Would your answer change if the error was not lie kely to affect other...
-
On April 15, 2021. Sampson Consulting provides services to a customer for $104,000. To pay for the services, the customer signs a three-year, 12% note. The face amount is due at the end of the third...
-
A chemical engineer wants to determine the feasibility of making ethanol (C 2 H 5 OH) by reacting water with ethylene (C 2 H 4 ) according to the equation Is this reaction spontaneous under standard...
-
Using the following data (at 25C),
-
Give a sequence of m MAKE-SET, UNION, and FIND-SET operations, n of which are MAKE-SET operations, that takes (m lg n) time when we use union by rank only.
-
Write out the form of the partial fraction decomposition of the function (See Example ). Do not determine the numerical values of the coefficients. (If the partial fraction decomposition does not...
-
Below is the actual assignment information. Here is where you will submit your event for approval. It is not graded, but you will need it to be marked complete in order to submit your paper, so...
-
3. (20 points) A researcher is interested in whether the phonics method of teaching reading is more or less effective than the sight method, depending on what grade the child is in. Twenty children...
-
Let A and B be the matrices given below: -5 9 -7 A= 8 -1 -3 B=9 6 -1 8 -1 -7. 0 Perform the following matrix operations and enter the entries below: -4A = A-4B = 5A-3B=
-
The product business can be isolated into four principal classes: programming administrations, framework administrations, open source and SaaS. The accompanying depicts the classifications of...
-
The gas-turbine cycle of a combined gas-steam power plant has a pressure ratio of 12. Air enters the compressor at 310 K and the turbine at 1400 K. The combustion gases leaving the gas turbine are...
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
What is the molality of acetone, C 3 H 6 O, in an aqueous solution for which the mole fraction of acetone is 0.112?
-
Calculate the standard Gibbs free energy for each of the following reactions: 2 HI(g), K = 54 at 700. K (a) H(g) + 1(g) (b) CCl3COOH (aq) + HO(1) = CClCO (aq) + H3O+ (aq), K = 0.30 at 298 K
-
Permanganate ions are powerful oxidizing agents used in water treatment facilities to remove metals, such as iron, and toxic and malodorous chemicals, such as H 2 S. If you are using permanganate...
-
Marigold industries had the following inventory transactions occur during 2020: 2/1/20 Purchase 51 units @ $46 cost/unit 3/14/20 purchase 98 units @ $49 cost/unit 5/1/20 purchase 68 units @ $53...
-
In this investment portfolio simulation, you and the bean counters, will invest and manage a fictitional amount of $ 1 , 0 0 0 , 0 0 0 during next three weeks. The simulation includes two fictitional...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...

Study smarter with the SolutionInn App


