Some organic compounds containing the C=O group can react with themselves in a process known as aldol
Question:
Some organic compounds containing the C=O group can react with themselves in a process known as aldol condensation.
The mechanism for this reaction in acidic solution is shown here.
Write the overall reaction, identify any intermediates, and determine the role of the hydrogen ion.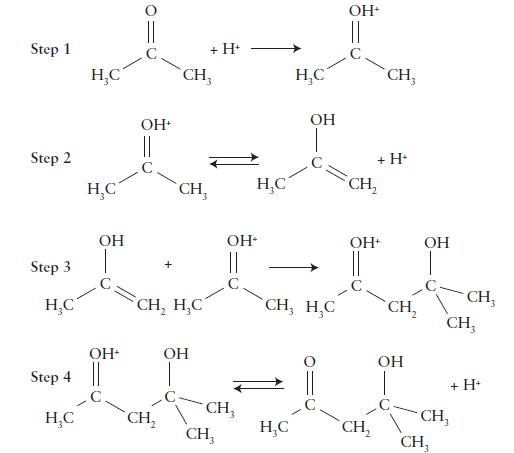
Transcribed Image Text:
Step 1 Step 2 Step 3 НС Step 4 Н С HC - НС www. OH ОН ОН || с CH, ans CH, HỌC CH₂ CH, + H+ OH ОН- || CH, CH₂ НС HC нс ОН CH, H, C OH+ CH₂ ОН CH₂ CH, + H CH2 OH OH CH CH₂ CH, + H+ CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
The image youve provided illustrates the steps of an aldol condensation mechanism in acidic conditions In this mechanism an aldehyde or ketone is acti...View the full answer

Answered By

Mishark muli
Having any assignments and any other research related work? worry less for I am ready to help you with any task. I am quality oriented and dedicated always to produce good and presentable work for the client once he/she entrusts me with their work. i guarantee also non plagiarized work and well researched work to give you straight As in all your units.Feel free to consult me for any help and you will never regret
4.70+
11+ Reviews
37+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The reaction of nitric oxide (NO(g)) with molecular hydrogen (H2(g)) results in the production of molecular nitrogen and water as follows: 2NO(g) + 2H 2 (g) N 2 O(g) + 2H 2 O(g) The experimentally...
-
Olde World Windows and Doors is a manufacturer of steel windows and doors for both residential and commercial applications. The following problems are decisions that senior management faces in...
-
Starting with benzene and methane, and suitable inorganic reagents, suggest how the following compound might be synthesized. See Exercise 37 for a description of how an alkyl group can be added to...
-
The Kay Company has the following Capital structure as at 31st March 2019. Based on Book Value Based on Market Value % Costs Debentures 300,000 330,000 ...
-
It is often useful to know how well your portfolio is diversified. Two measures have been suggested: a. The variance of the returns on a fully diversified portfolio as a proportion of the variance of...
-
Cash management versus liquidity management what is the difference between cash management and liquidity management?
-
Describe an investment portfolio and how youd go about developing, monitoring, and managing a portfolio of securities.
-
Reitmans (Canada) Limited is a leading Canadian retailer that operates more than 900 stores under the Reitmans, Smart Set, RW & Co., Thyme Maternity, Penningtons, and Addition Elle banners. The...
-
Suppose that the index model for stocks A and B is estimated from excess returns with the following results: R A = 3 . 0 0 % + 1 . 0 5 R M + e A R B = - 1 . 2 0 % + 1 . 2 0 R M + e B M = ( ) A Assume...
-
The hydrolysis of sucrose (C 12 H 22 O 11 ) produces fructose and glucose: C 12 H 22 O 11 (aq) + H 2 O(l) C 6 H 12 O 6 (glucose, aq) + C 6 H 12 O 6 (fructose, aq). Two mechanisms are proposed for...
-
The rate law of the reaction 2 NO(g) + 2 H 2 (g) N 2 (g) + 2 H 2 O(g) is Rate = kr[NO] 2 [H 2 ], and the mechanism that has been proposed is (a) Which step in the mechanism is likely to be rate...
-
Given the set {U1,.........,Un} of iid uniform (0;T) random variables, we defince Xk = smallk(U1,...........,Un) As the kth "smallest" element of the set. That is , X1 is the minimum element, x2 is...
-
According to the College Board website, the scores on the math part of the SAT (SAT-M) in a certain year had a mean of 507 and a standard deviation of 111. Assume that SAT scores follow a normal...
-
Pay and incentive programs are being used both for knowledge workers and in non-knowledge worker occupations. In every industry, from restaurants to construction and low-tech manufacturing, companies...
-
Closet International invested in an equipment in 2019 with an initial cost of $598,000. It falls under asset class 8 with a CCA rate of 20%. The equipment was sold in 2021 for $260,000. Calculate the...
-
Question 4 (30 Marks) A 12-ply Kevlar/Epoxy composite beam with layup [0/90 / 0 1s is loaded in 3-point bending, as shown in Figure Q4. The beam has a length, L of 100mm, a width, b of 25mm and a...
-
Scenario: You have been working in a community service sector for two years. However, you always find evaluating your own performance challenging. Your Supervisor has also identified that you do not...
-
Use the data in APPLE.RAW for this exercise. These are telephone survey data attempting to elicit the demand for a (fictional) "ecologically friendly" apple. Each family was (randomly) presented with...
-
True & False The basis of an asset must be reduced by the depreciation allowable, 2. Adjusted gross income (AGI) is the basis for a number of phase-outs of deductions. 3. A change to adjusted gross...
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
The fundamental vibrational frequencies for 1 H 2 and 2 D 2 are 4401 and 3115 cm 1 , respectively, and De for both molecules is 7.667 10 19 J. Using this information, calculate the bond energy of...
-
A simulated infrared absorption spectrum of a gas-phase organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...

Study smarter with the SolutionInn App


