The four substances HCl, I 2 , HI, and Cl 2 are mixed in a reaction vessel
Question:
The four substances HCl, I2, HI, and Cl2 are mixed in a reaction vessel and allowed to reach equilibrium in the reaction 2 HCl(g) + I2(s) ⇌ 2 HI(g) + Cl2(g). Certain changes (which are specified in the first column in the following table) are then made to this mixture. Considering each change separately, state the effect (increase, decrease, or no change) that the change has on the original equilibrium value of the quantity in the second column (or K, if that is specified). The temperature and volume are constant.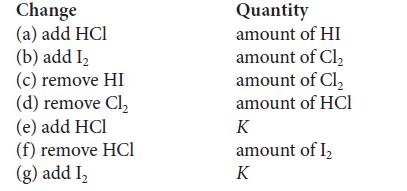
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





