Use data from Table 4C.1 to calculate the vapor pressure of methanol at 25.0 C. TABLE 4C.1
Question:
Use data from Table 4C.1 to calculate the vapor pressure of methanol at 25.0 °C.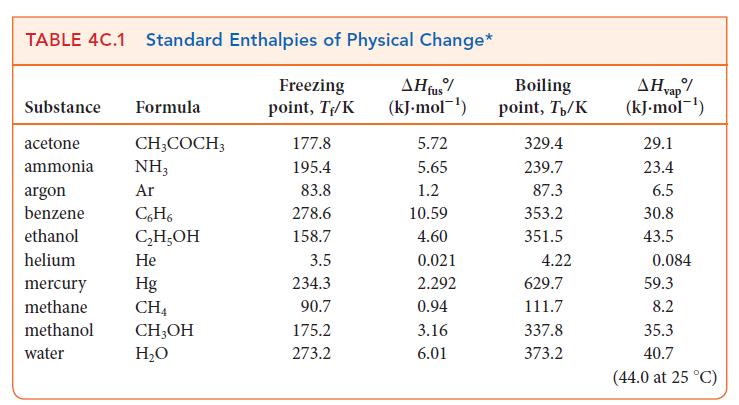
Transcribed Image Text:
TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ.mol-¹) point, T₁/K Substance Formula acetone ammonia argon benzene ethanol helium CH3COCH3 NH3 Ar C6H6 C₂H₂OH He Hg CH4 mercury methane methanol CH₂OH water H₂O 177.8 195.4 83.8 278.6 158.7 3.5 234.3 90.7 175.2 273.2 5.72 5.65 1.2 10.59 4.60 0.021 2.292 0.94 3.16 6.01 Boiling point, T/K 329.4 239.7 87.3 353.2 351.5 4.22 629.7 111.7 337.8 373.2 AH vap/ (kJ.mol-¹) 29.1 23.4 6.5 30.8 43.5 0.084 59.3 8.2 35.3 40.7 (44.0 at 25 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
P 250 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
You are a chemical engineer at a processing plant for soft drinks. You have been asked to determine whether a particular solution will have a significantly different vapor pressure from pure water....
-
Calculate the vapor pressure at 25 C of a solution containing 99.5 g sucrose (C 12 H 22 O 11 ) and 300.0 mL water. The vapor pressure of pure water at 25 C is 23.8 torr. Assume the density of water...
-
Use data from Table 4C.1 to calculate the vapor pressure of mercury at 275 K. TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ. mol) point, T/K Substance Formula acetone...
-
Tell whether the given side lengths of a triangle can represent a right triangle. 36, 48, and 60
-
Describe three methods suitable for the separation of air into nitrogen and oxygen.
-
In straight-bevel gearing, there are some analogs to Eqs. (1444) and (1445). If we have a pinion core with a hardness of (HB) 11 and we try equal power ratings, the transmitted load Wt can be made...
-
(Segment Reporting) You are compiling the consolidated financial statements for Vender Corporation International. The corporations accountant, Vincent Price, has provided you with the following...
-
PrimeTime Sportswear is a custom imprinter that began operations six months ago. Sales have exceeded management's most optimistic projections. Sales are made on account and collected as follows: 60%...
-
0 Required information The Foundational 15 (Algo) (LO6-1, L06-3, L06-4, LO6-5, L06-6, L06-7, LO6-8) The following information applies to the questions displayed below.) Oslo Company prepared the...
-
Billingham Packaging is considering expanding its production capacity by purchasing a new machine, the XC-750. The cost of the XC-750 is $2.75 million. Unfortunately, installing this machine will...
-
The density of a 5.00% by mass K 3 PO 4 aqueous solution is 1.043 g cm 3 . Determine (a) The molality; (b) The molarity of potassium phosphate in the solution.
-
Most reactions proceed faster at higher temperatures, and many industrial processes are carried out at high temperatures. However, for exothermic reactions increasing the temperature reduces the...
-
Using a word of 3 bits, list all the possible signed binary numbers and their decimal equivalents that are representable in a) Signed magnitude b) Ones complement c) Twos complement
-
How can you filter on a particular data field in Cognos Analytics? 0 / 1 point Type in the name of the field in the 'Filters' area at the top of the page. Drag the data field to the 'All tabs' area...
-
Project management is fundamental to project success and includes a number of critical success factors including support of top management, use of effective communication channels and rapid feedback,...
-
BIO 189: what are some of the ethical issues regarding his results? why were his results rejected by the scientific community? choose two components below that were either flawed or completely absent...
-
REQUIRED: Prepare Cost of Production per department. Problem 5 ABM Company uses two departments to produce a product. The following data were taken from the books for the month of January, 2019....
-
Can you describe how Toyota responds to changes in market fluctuations, and plot the supply and demand curves indicating managerial economic principles (i.e. price ceilings/floors, shortage/surplus,...
-
After you restore files following an incident, users complain that some of their data files are missing. What might have happened?
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
Using 2-propanol as your only source of carbon, show how you would prepare 2-methyl-2-pentanol.
-
A bottle at 325 K contains an ideal gas at a pressure of 162.5 10 3 Pa. The rubber stopper closing the bottle is removed. The gas expands adiabatically against P external = 120.0 10 3 Pa, and some...
-
Predict the product and draw the mechanism for each of the following reactions: a. b. c. 1) LAH 2) H20 1) LAH 2) H20
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...
-
Regarding Enron, this was a company that resulted in the creation of the Sarbanes-Oxley Act and many reforms to the accounting profession. Research the company and answer the following...

Study smarter with the SolutionInn App


