Balance each chemical equation. SiO (s) + Ca(HCO3)2(aq) CoS3(s) + NH4NO3(aq) a. CO(g) + CaSiO3(s) + HO(1)
Question:
Balance each chemical equation.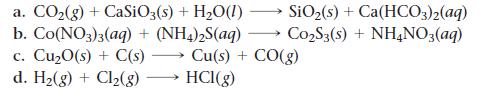
Transcribed Image Text:
SiO₂ (s) + Ca(HCO3)2(aq) Co₂S3(s) + NH4NO3(aq) a. CO₂(g) + CaSiO3(s) + H₂O(1) b. Co(NO3)3(aq) + (NH4)2S(aq) c. Cu₂0(s) + C(s) Cu(s) + CO(g) d. H₂(g) + Cl₂(g) HCI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a 2 COg CaSiO3s HO1 SiO ...View the full answer

Answered By

AJIN KURIAKOSE
I HAVE ELECTRONICS ENGINEERING DEGREE..AND MY AREA OF INTEREST IS MATHEMATICS,CONTROL SYSTEM,NETWORK,DIGITAL
4.70+
21+ Reviews
32+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In Exercises 1-3, balance the chemical equation for each reaction.
-
The scatterplot shows the median weekly earning (by quarter) for men and women in the United States for the years from 2005 through 2017. The correlation is 0.974. a. Use the scatterplot to estimate...
-
Total current assets TOTAL ASSETS SHAREHOLDERS' EQUITY AND LIABILITIES: Shareholders' Equity: Preferred stock Common stock: Tk. 10 par 100,000 shares Share Premium 1690 2070 3060 3520 100 100 1000...
-
Consider the following propositions: p: 2 is the smallest prime q: 6 is a perfect square Represent the proposition "2 is the smallest prime but 6 is not a perfect square" using logical connectives....
-
In the table, find the Treasury bond that matures in May 2033. What is the asked price of this bond in dollars? If the bid-ask spread for this bond is two ticks, what is the bid price in dollars?
-
The air-release flap on a hot-air balloon is used to release hot air from the balloon when appropriate. On one hot-air balloon, the air release opening has an area of 0.5 m2, and the filling opening...
-
Build an Excel spreadsheet to compute a discount rate of 8 percent per year for each requirement. Required a. Present value of $(5,000) now b. Present value of $1,500 one year from now c. Present...
-
Sierra Foxtrot Airport called for tenders for supplies of green seed for its runway surrounds, with a closing date of 1 June. The following tenders were submitted: Green Grow hand-delivered its...
-
An insurance company has an obligation to pay the medical costs for a claimant Annual claim costs today are 35,000, and medical inflation is expected to be 2.5% per year. The claimant will receive 10...
-
Kate, Tina and Naomi - leading Gold coast civil engineering, construction and finance consultants - set up their own firm, KTN Consulting Pty Ltd (KTN). The business expanded rapidly. A joint venture...
-
Write the balanced chemical equation for the reaction of aqueous potassium hydroxide with aqueous iron(III) chloride to form solid iron(III) hydroxide and aqueous potassium chloride.
-
Write the balanced chemical equation for the reaction of aqueous sodium carbonate with aqueous copper(II) chloride to form solid copper(II) carbonate and aqueous sodium chloride.
-
Sumter Pumps Corporation, a manufacturer of industrial pumps, reports the following results for the year ended January 31, 2016: Retained earnings, February 1, 2015 . . . . . . . . . . . . . . . . ....
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Harvey Auto Parts purchased a new crane on September 1 for $35,000, paying $10,000 cash and signing a 7%, 12-month note for the remaining balance, interest to be paid at maturity. The crane is...
-
e4(k+1) Find the sum of the series. k = 1 8
-
Carla Vista Corp. sponsors a defined benefit pension plan for its employees. On January 1, 2025, the following balances relate to this plan Plan assets $489,900 Projected benefit obligation 616,700...
-
Question 2 of 8 Shirts were purchased for $12.50 each and were marked up by $18.75. During Christmas, they were discounted by $6.85 per shirt. a. What was the rate of markdown? % Round to two decimal...
-
A pea plant that is dwarf with green, wrinkled seeds was crossed t' a true-breeding plant that is tall with yellow, round seeds. The F1 generation was allowed to self-fertilize. What types of...
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Consider the reaction FeO(s) + CO(g) Fe(s) + CO 2 (g) for which K P is found to have the following values: a. Calculate ÎG o R , ÎS o R , and ÎHR???? for this reaction at...
-
If K P is independent of pressure, why does the degree of dissociation in the reaction Cl 2 (g) 2Cl(g) depend on pressure?
-
How does the total number of moles in the reaction system change as T increases? H 2 (g) + Cl 2 (g) 2HCl(g) at equilibrium. Assume ideal gas behavior.
-
Data Table The adjusted trial balance of Emes Real Estate Appraisal at June 30, 2024, follows: Click the icon to view the adjusted trial bsanen) Read the requirement Um Requirement 1. Prepare the...
-
Suppose you are the CFO of a large firm. Would you prefer to show company earnings based on the all-inclusive format or the current operating performance format, present GAAP notwithstanding? Explain...
-
Using an Aging Schedule to Account for Bad Debts Sparkle Jewels distributes fine stones. It sells on credit to retail jewelry stores and extends terms that require the stores to pay in 60 days. For...

Study smarter with the SolutionInn App


