Estimate the value of the equilibrium constant at 525 K for each reaction in Problem 73. Problem
Question:
Estimate the value of the equilibrium constant at 525 K for each reaction in Problem 73.
Problem 73
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction.![]()
Appendix IIB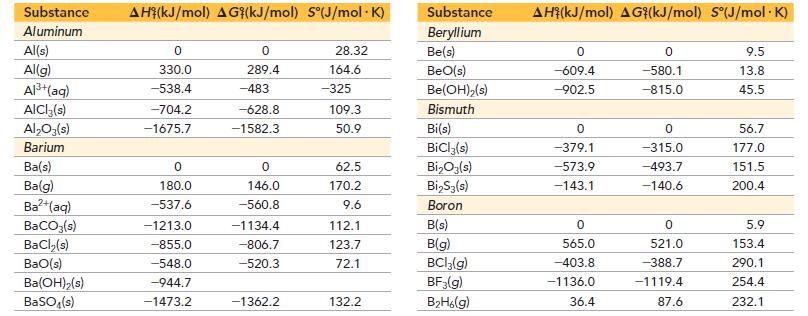
Transcribed Image Text:
a. 2 CO(g) + O₂(g) = 2 CO₂(g) b. 2 H₂S(g) = 2 H₂(g) + S₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a 190...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H f for BrCl is 14.6 kJ/mol.) Problem 74 Use data from Appendix IIB to calculate the equilibrium constants at...
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
What future directions may be interesting for comparative studies in corporate governance?
-
Explain how an activity-based budget is prepared.
-
The accompanying diagram illustrates the U.S. domestic demand curve and domestic supply curve for beef. The world price of beef is PW. The United States currently imposes an import tariff on beef, so...
-
C2.9. Why are dividends notan expense in the income statement?
-
Use the methods of descriptive statistics to learn about the customers who visit the Heavenly Chocolates website. Include the following in your report. 1. Graphical and numerical summaries for the...
-
ames Manufacturing had the following information available for July: Actual Results Flexible Budget Variance Flexible Budget Sales Activity Variance Master Budget Units 12,000 ? 3,000 U ? Sales...
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P ICl = 2.55 atm; P I2 = 0.325 atm; P Cl2 = 0.221...
-
Describe the solubility of CaF 2 in each solution compared to its solubility in water. a. In a 0.10 M NaCl solution b. In a 0.10 M NaF solution c. In a 0.10 M HCl solution
-
Blair sold the following stocks in 2017: 200 shares of Dearborn Investments purchased May 15, 2016, for $3,050 and sold on January 9, 2017 for $4,135; and 40 shares of State Street Investments,...
-
Moving Inc. wants to develop an activity flexible budget for the activity of moving materials. Moving Inc. uses forklifts to move materials from receiving to storeroom and then to production. The...
-
We are in the tail end of Quarter 3 earnings reporting season in the U.S. markets. Roughly 60 percent of companies that have reported their Q3 earnings so far have reported negative earnings relative...
-
Below is a running shock tube illustration. 0.1 0.0 | 0.0 4 4 Diaphragm 1 0.5 Image: Shock tube Initial setup 1 3 2 1 Expansion Head Expansion Tail Slip Shock Surface Image: Running Shock Tube...
-
As you may remember, Holiday Tree Services, Inc. (HTS) has recently entered into a contract with Delish Burger (Delish), whereby HTS is to supply and decorate a Christmas tree in each of Delish...
-
Understanding various types of leadership styles is important in order to determine personal leadership styles. Reflection: Answer both Compare and contrast 2 leadership styles. State the...
-
In advance of the recent increase in the U.S. minimum wage rate, the government of the state of Arizona decided to boost its own minimum wage by an additional $1.60 per hour. This pushed the wage...
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
Redraw the ray diagram for a compound microscope (Fig. 5.110), but this time treat the intermediate image as if it were a real object. This approach should be a bit simpler. Fig. 5.110 Exit pupil fe...
-
Consider a thin positive lens L 1 , and using a ray diagram, show that if a second lens L 2 is placed at the focal point of L 1 , the magnification does not change. Thats a good reason to wear...
-
Draw a ray diagram locating the images of a point source as formed by a pair of mirrors at 90? (Fig. P.5.61a). Now create a ray diagram locating the images of the arrow shown in Fig. P.5.61b. Fig...
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


