What mass of ethylene glycol (C 2 H 6 O 2 ), in grams, must be added
Question:
What mass of ethylene glycol (C2H6O2), in grams, must be added to 1.0 kg of water to produce a solution that boils at 105.0 °C?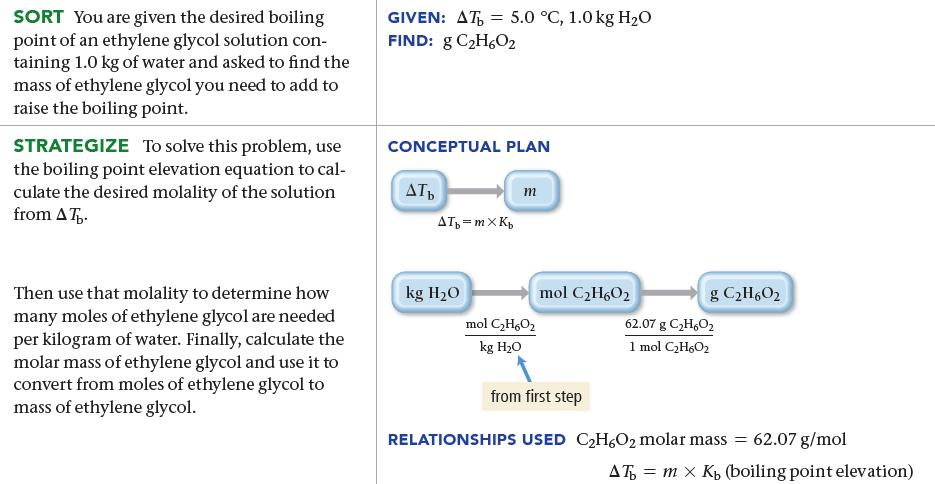
Transcribed Image Text:
SORT You are given the desired boiling point of an ethylene glycol solution con- taining 1.0 kg of water and asked to find the mass of ethylene glycol you need to add to raise the boiling point. STRATEGIZE To solve this problem, use the boiling point elevation equation to cal- culate the desired molality of the solution from AT. Then use that molality to determine how many moles of ethylene glycol are needed per kilogram of water. Finally, calculate the molar mass of ethylene glycol and use it to convert from moles of ethylene glycol to mass of ethylene glycol. GIVEN: AT = FIND: g C₂H602 CONCEPTUAL PLAN AT 5.0 °C, 1.0 kg H₂O ATt=mXK₂ kg H₂O m mol C₂H6O₂ kg H₂O mol C₂H60₂ from first step RELATIONSHIPS USED g C₂H6O2 62.07 g C2₂H₂O₂ 1 mol C₂H₂O₂ C₂H6O₂ molar mass = 62.07 g/mol AT = mx K, (boiling point elevation)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Tb m x Kb 977 m 10 k...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose there are only two countries in the world, Foreign and Home. Foreign is a large country while Home is small country. Currently, Home runs a huge trade deficit. Concerning about the country's...
-
How many grams of ethylene glycol (C2H6O2) must added to 1.00 kg of water to produce a solution that freezes at - 5.00 oC?
-
What volume of ethylene glycol (C 2 H 6 O 2 ), a nonelectrolyte, must be added to 15.0 L water to produce an antifreeze solution with a freezing point of -25.0 C? What is the boiling point of this...
-
1) Write a generic function to integrate y(x)dx with the following format function I integrator (x, y, method) where x is a vector and y is a matrix whose columns y(:,j) are vectors of the same...
-
Menotti Company produces two types of space heaters (regular and super). Both pass through two producing departments: fabrication and assembly. It also has a materials handling department that is...
-
Prove Part 3 of Theorem 1.14 using only Definition 1.6. That is, prove that for any non-negative integer \(k\), \[\frac{d}{d x} H_{k}(x)=k H_{k-1}(x)\] Do not use the result of Part 1 of Theorem...
-
Calvin Consulting initially records prepaid and unearned items in income statement accounts. Given this QS 3-1 companys accounting practices, which of the following applies to the preparation of...
-
Northern Gas wants to move its sales order system to the Internet. Under the proposed system, gas stations and other merchants will use a secure site to check the availability and current price of...
-
Moe is deciding whether or not to introduce a new drink, the Flaming Moe. The Flaming Moe is made up of several ingredients which cost $1.25 per drink. Moe expects to sell the drink for $4. He...
-
1 to 3. First, post the unadjusted balances from the unadjusted trial balance that was given and the adjusting entries that were made in Problem 2-3 into the appropriate T-accounts (on the T-accounts...
-
What are the three steps involved in evaluating the enthalpy changes associated with solution formation?
-
What mass of glucose (C 6 H 12 O 6 ) should be dissolved in 10.0 kg of water to obtain a solution with a freezing point of -4.2 C? a) 0.023 kg b) 4.1 kg c) 0.41 kg d) 14.1 kg
-
Foymount Industries Inc. borrowed $800,000 from Development Bank to finance the purchase of equipment costing $650,000 and to provide $150,000 in cash. The legal documentation states that the loan...
-
2.7 The percent impedance of a transformer is typically determined by a short circuit test. In such a test, the secondary of the transformer is shorted and the voltage on the primary is increased...
-
Describe the ideal target market for Farmer's Fridge vending machines. Explain why this target market is viable for the company. 2. Describe Farmer's Fridge target market using the four segmentation...
-
Global sustainable development is strongly linked with the environment. Describe any study or simulation of the sustainability of global development and its main conclusions.
-
The issues in the software development area of Informational Systems have grown into a genuine workplace conflict. One of the issue is interdependencethe Millennials want to schedule their work on...
-
At what stage of the system development project (for example feasibility study, requirement analysis, etc) would a prototype be useful as means of reducing the following uncertainties? two Scenario...
-
(a) Briefly cite the main differences between ionic, covalent, and metallic bonding. (b) State the Pauli exclusion principle.
-
Under what conditions is the following SQL statement valid?
-
Balancing act. The tightrope walker in Figure P4.8 gets tired and decides to stop for a rest. During this rest period, she is in transnational equilibrium. She stops at middle of the rope and finds...
-
A system of cables is used to support a crate of mass m = 45 kg as shown in Figure P4.7. Find the tensions in all three cables. Figure P4.7 T3 T1 K60 T2
-
Consider again the wedge in Question 6, but now assume a block is placed onto it as shown in Figure Q4.7. There is again no friction between the wedge and the table, and there is also no friction...
-
SECTION B: ANALYSIS OF FINANCIAL STATEMENT (USING INTEL CORP STATEMENTS ABOVE ANSWER QUESTIONS BELOW) 1. Compute the major ratios related to the firms liquidity situation and comment on the firms...
-
Case 9-47 Comprehensive Master Budget; Short-Term Financing; Acquisition of Robotic Equipment (LO 9-2, 9-3, 9-5, 9-6) Skip to question [The following information applies to the questions displayed...
-
Briefly compare and contrast Return on Investment with Residual Income. Be sure to discuss the advantages and disadvantages of each. When might it be more appropriate to use one method over another.

Study smarter with the SolutionInn App


