Use the Nernst equation to calculate the cell potentials of the following cells at 298 K. (a)
Question:
Use the Nernst equation to calculate the cell potentials of the following cells at 298 K.
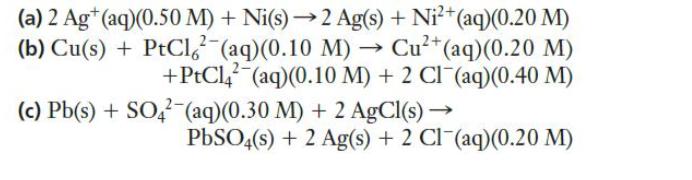
Transcribed Image Text:
(a) 2 Ag+ (aq)(0.50 M) + Ni(s)→2 Ag(s) + Ni²+ (aq)(0.20 M) (b) Cu(s) + PtCl (aq) (0.10 M)→ Cu²+ (aq) (0.20 M) +PtCl (aq)(0.10 M) + 2 Cl(aq)(0.40 M) (aq)(0.30 M) + 2 AgCl(s) → (c) Pb(s) + SO4 PbSO4(s) + 2 Ag(s) + 2 Cl(aq)(0.20 M)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a E 10...View the full answer

Answered By

SUMAN DINDA
I LIKE TO TEACH STUDENTS. SO, I START MYSELF AS A PRIVATE TUTOR. I TEACH STUDENTS OF DIFFERENT CLASSES. I HAVE ALSO DONE BACHELOR OF EDUCATION DEGREE(B.ED). DURING THIS COURSE I HAD TO TEACH IN A SCHOOL. SO I HAVE A GOOD EXPERIENCE IN TEACHING.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Determine the evaluative criteria that your target market(s) would use when choosing between alternative brands?
-
(a) Use the Nernst equation to write the spontaneous chemical reaction that occurs in the cell in Demonstration 13-1. (b) If you use your fingers as a salt bridge in Demonstration 13-1, will your...
-
Company D is considering an investment project which has the following cash flows: Year 0: -1,200,000 Year 1: 252,000 Year 2: 340,000 Year 3: -200,000 Year 4: 580,000 Year 5: 700,000 Year 6: -200,000...
-
Introduction to Accounting An Integrated Approach EighthEdition Chapter 5 P5.10 P5.10 Engelhaupt Company is considering a switch to JIT. It has gathered the following data: In addition, Engelhaupt...
-
What is the first step in the writing process for a persuasive message, and why is this step important?
-
A moving helper company gave Mike these two quotes. Use a system of equations to determine the hourly rates for loading/unloading and packing / unpacking? 3 hours of loading/unloading 2 hours of...
-
3 Where would you place each of the following sales jobs on the order-taker/order-getter continuum shown below? (a) Burger King counter clerk, (b) automobile insurance salesperson, (c) Hewlett-...
-
Reid & Company uses the periodic inventory system. On January 1, it had an inventory balance of $250,000. During the year, it made $613,000 of net purchases. At the end of the year, a physical...
-
Please help me with Dec 3 1 . TIA
-
We noted that a tin-plated steel can corrodes more quickly than an unplated steel can. In cases of galvanic corrosion, one cannot expect standard conditions. Suppose that you want to study the...
-
Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells. (a) (b) (c) Fe(s) | Fe+ (aq) || Hg2+ (aq) | Hg()
-
Describe the significance of the shift from transaction-based marketing to relationship marketing. When does relationship building begin?
-
Why do you think diversity is important to organizations and what can a do to increase diversity in leadership? What is Servant Leadership? How can you apply this in your life? What is effective team...
-
How do you envision overcoming any potential resistance or skepticism from your colleagues in the vet tech field as you introduce these transformative strategies, and what steps do you think will be...
-
Managers encourage employees to do misleading activities such as speak falsehood and deceive customers which is clearly visible in the statement in the case " Sales are everything" wherein an...
-
Your Topic is "Why do you think there are so few people who succeed at both management and leadership? Is it reasonable to believe someone can be good at both?" Locate two to three articles about...
-
Explain the various benefits associated with professional networking. Also, expand on your answers how those would benefit you personally. PLEASE DO FAST AND CORRECT need correct answer
-
What do you think are the dangers of this type of scheme for McDonalds?
-
Write a declaration for each of the following: a. A line that extends from point (60, 100) to point (30, 90) b. A rectangle that is 20 pixels wide, 100 pixels high, and has its upper-left corner at...
-
The resulting stress distribution along section AB for the bar is shown. From this distribution, determine the approximate resultant axial force P applied to the bar. Also, what is the stress...
-
The resulting stress distribution along section AB for the bar is shown. From this distribution, determine the approximate resultant axial force P applied to the bar. Also, what is the stress...
-
The member is to be made from a steel plate that is 0.25 in. thick. If a 1-in. hole is drilled through its center, determine the approximate width w of the plate so that it can support an axial force...
-
explain the concept of Time Value of Money and provide and example. In addition to your discussion, please explain the differences between Stocks and Bonds
-
Wildhorse Inc. has just paid a dividend of $3.80. An analyst forecasts annual dividend growth of 9 percent for the next five years; then dividends will decrease by 1 percent per year in perpetuity....
-
Jenny wanted to donate to her alma mater to set up a fund for student scholarships. If she would like to fund an annual scholarship in the amount of $6,000 and her donation can earn 5% interest per...

Study smarter with the SolutionInn App


