Calculate the enthalpy change for using the thermochemical equations C2H,(g) + HO(0) CHOH(0) = ?
Question:
Calculate the enthalpy change for![]()
using the thermochemical equations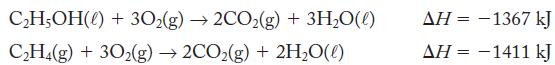
Transcribed Image Text:
C2H,(g) + HO(0) → CHOH(0) ΔΗ = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)

Answered By

David Ngaruiya
i am a smart worker who concentrates on the content according to my clients' specifications and requirements.
4.50+
7+ Reviews
19+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Th e chemical industry converts hydrocarbons of low molecular mass to larger and more useful compounds. Calculate the change in enthalpy for the synthesis of cyclohexane (C 6 H 12 ), a compound used...
-
Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation of ethylene to form ethane. Calculate the enthalpy change for Use the following...
-
The chapter introduction introduced the following reaction as one chemical reaction used to launch the space shuttle. Calculate the mass of aluminum required to generate 60,500 kJ energy (enough to...
-
Dylan Flaherty, marketing clerk for TipTop Marketing Agency, recorded the following information for last year: He would like to be able to estimate customer service costs using the number of...
-
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100-105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures for pure benzene vapor on silica gel, having the following...
-
In 2 0 2 4 , the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $ 1 0 , 0 0 0 , 0 0 0 . The road was completed in 2 0 2 6 . Information related...
-
Distinguish between the terms "measured" and "denominated." LO6
-
You are working as a Road Design Engineer for Vic Roads. Your Team Leader asks you to combine both horizontal and vertical alignment. If the length of a vertical sag curve is 260 m, how much should...
-
ABC Company Inc. informa su contabilidad en base devengada. Qu deben hacer si no han recibido una factura trimestral antes de fin de mes? * Nada, espera a que llegue la factura y regstrala cuando...
-
Ethyl alcohol (ethanol) can be produced by the fermentation of sugars derived from agricultural products such as sugarcane and corn. Some countries without large petroleum and natural gas...
-
Differentiate between kinetic energy and potential energy.
-
A 50.0 g-sample of acid takes 46.4 mL of 0.500 M NaOH solution to neutralize it. Assume the same amount of heat is given off as in Example 5.6. (a) Calculate the enthalpy change for the...
-
The flowtime of the last job in a single work center's schedule is 7 days. What is the makespan of this schedule?
-
Which one of the following is not a part of the Deployment phase of a machine learning development project? Explain what phase(s) address this issue, and why then? Training end users to incorporate...
-
Assist with the following discussion: Topic Discussion #1B: The first half of the term is devoted to leaders preparing themselves for leadership. Peter Senge and his coauthors discuss in The Dawn...
-
You are managing an employee who is not a self-starter, and thus you need to devise a plan to effectively lead this employee. Draft a one page (Times New Roman 12) single space response (plus title...
-
Ontario's minister of training, colleges and universities defended changes to post-secondary education on Monday, saying recently announced decisions are all about the making the system more...
-
"The power of globalization is not about leveraging economies of scale. It's about leveraging economies of knowledge and coordination figuring out how not to reinvent the wheel everywhere you do...
-
What are the current relational DBMSs that dominate the market? Pick one that you are familiar with and show how it measures up based on the criteria laid out in Section 10.2.3?
-
Estimate a range for the optimal objective value for the following LPs: (a) Minimize z = 5x1 + 2x2 Subject to X1 - x2 3 2x1 + 3x2 5 X1, x2 0 (b) Maximize z = x1 + 5x2 + 3x3 Subject to X1 + 2x2 +...
-
Draw the enol of each of the following compounds, and identify whether the enol exhibits a significant presence at equilibrium. Explain. (a) (b) (c) (d)
-
Ethyl acetoacetate has three enol isomers. Draw all three.
-
Draw the enolate that is formed when each of the following compounds is treated with LDA. (a) (b) (c) (d)
-
An 8%, 30-year semi-annual corporate bond was recently being priced to yield 10%. The Macaulay duration for this bond is 10.2 years. What is the bonds modified duration? How much will the price of...
-
Question 7 of 7 0/14 W PIERDERY Current Attempt in Progress Your answer is incorrect Buffalo Corporation adopted the dollar value LIFO retail inventory method on January 1, 2019. At that time the...
-
Cost of debt with fees . Kenny Enterprises will issue a bond with a par value of $1,000, a maturity of twenty years, and a coupon rate of 9.9% with semiannual payments, and will use an investment...

Study smarter with the SolutionInn App


