Calculate the value of the solubility product constant for Cd(OH) 2 from the half-cell potentials.
Question:
Calculate the value of the solubility product constant for Cd(OH)2 from the half-cell potentials.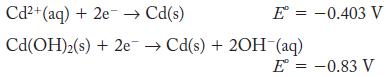
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The question presents us with halfcell potentials for two reactions involving cadmium To calculate t...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2B and the fact that, for the half-reaction F 2 (g) + 2 H + (aq) + 2 e 2 HF(aq), E = 13.03 V, to calculate the value of K a for HF. 2B STANDARD POTENTIALS AT 25 C...
-
As in Example 6L.1, you are planning to use a Daniell cell to power a model electric car. However, you find that you do not have standard solutions available. You have only dilute solutions, and you...
-
Calculate the solubility product constant for copper(II) iodate, Cu(IO3)2. The solubility of copper(II) iodate in water is 0.13 g/100 mL.
-
What does 'buying local' mean to you? How do you determine what 'local' means? Are there limits to buying local here in PEI? Is buying local a priority for you when making purchases? How do you...
-
Determine F12 and F21 for the following configurations using the reciprocity theorem and other basic shape factor relations. Do not use tables or charts. (a) Long duct (b) Small sphere of area A1...
-
Use the Laplace, Wald and Savage decision criteria to select alternatives in the following matrices. What results would you get for other decision criteria? (a) Cost matrix (b) Gainsmatrix Event...
-
Explain the accounting for long-term notes payable.
-
The following scenarios describe situations currently facing companies. For each scenario, indicate whether a standard costing system would be beneficial in that situation or not and explain why or...
-
What is 1 plus 1? Options: A) 2 B) 11 C) Both D) None of the above. Wrong answer= Report = Downvote No entry for AI-generated answers.
-
Calculate the value of the solubility product constant for PbSO 4 from the half-cell potentials.
-
Using the voltaic cell in Exercise 18.64 , a voltage of 0.425 V was measured when the cell was placed in a solution of unknown ion concentrations. What is the ratio [Cd 2+ ]/[Ag + ] 2 in this...
-
Add MR and MC columns to Table in the chapter and find the profit-maximizing output level using the MR and MC approach. When calculating MR and MC, dont forget to divide ÎTR and ÎTC by...
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
10 Count scallops cost $12.97 per pound. How much do they cost for each? A Wagyu Beef New York Strip costs $14 per pound and weighs 15 pounds. The useable yield is 12.5 pounds. How many 12 ounce...
-
How do coordinating agencies differ in a crisis, disaster, and an emergency ?Explain
-
How do we manage and respond to customer feedback and reviews to maintain a positive brand reputation? Explain with the help of examples.
-
In the Lewis structure shown here, A, D, E, Q, X, and Z represent elements in the first two rows of the periodic table (H--- Ne). Identify all six elements so that the formal charges of all atoms are...
-
Archangel Corporation prepared the following variance report. Instructions Fill in the appropriate amounts or letters for the question marks in the report. ARCHANGEL CORPORATION Variance...
-
A barge is 60 ft long, 20 ft wide, and 8 ft deep. When empty, it weighs 210 000 lb, and its center of gravity is 1.5 ft above the bottom. Is it stable when floating in water?
-
If the barge in Problem 5.57 is loaded with 240 000 lb of loose coal having an average density of 45 lb/ft 3 , how much of the barge would be below the water? Is it stable? In Problem A barge is 60...
-
A piece of cork having a specific weight of 2.36 kN/m 3 is shaped as shown in Fig. 5.32. (a) To what depth will it sink in turpentine (sg = 0.87) if placed in the orientation shown? (b) Is it stable...
-
Roberson Corporation uses a periodic inventory system and the retail inventory method. Accounting records provided the following information for the 2018 fiscal year: Cost Retail Beginning inventory...
-
There are three basic approaches to CVP analysis - equation approach, contribution margin approach, and the contribution ratio margin approach. How are these approaches similar and how do they...
-
There are six farmers in Great Britain with access to government land to graze their cows for free. They all must share the land. Each farmer has an individual incentive to put as many of his cows on...

Study smarter with the SolutionInn App


