Hydrazine, N 2 H 4 , is used as a fuel in some rockets: What is the
Question:
Hydrazine, N2H4, is used as a fuel in some rockets: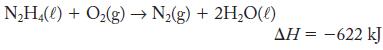
What is the enthalpy change if 110.0 g N2H4 reacts with excess oxygen?
Transcribed Image Text:
N₂H4(l) + O₂(g) → N₂(g) + 2H₂O(l) ΔΗ = −622 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
The given reaction is N2H41 O2g N2g 2H2O1 AH 622 kJ First we need to fin...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Hydrazine (N 2 H 4 ) is used as a fuel in liquid-fueled rockets. When hydrazine reacts with oxygen gas, nitrogen gas and water vapor are produced. Write a balanced equation and use bond energies from...
-
The steering rockets in space vehicles use N 2 O 4 and a derivative of hydrazine, 1,1-dimethylhydrazine (Study Question 5.86). This mixture is called a hypergolic fuel because it ignites when the...
-
What is a conversion rate and why is it so important to marketers? Provide an example of how an organization can improve this rate.
-
What is the smallest (most negative) 32-bit binary number that can be represented with (a) Unsigned numbers? (b) Twos complement numbers? (c) Sign/magnitude numbers?
-
Fifty-thousand pounds per hour of a 20 wt% aqueous solution of NaOH at 120F is to be fed to an evaporator operating at 3.7 psia, where the solution is concentrated to 40 wt% NaOH. The heating medium...
-
Cost Profit Volume Analysis You are helping a luxury handmade soap company conducting CVP analysis. Complete the table below with given information. Moisturiser Care Soap Balanced & Mild Soap Goat...
-
Financial Leverage (LO6) Following are data from McClellan Company and McDonough Company for 2012: McClellan McDonough Nein COMOmmtner nn ree ee ool cies va Dh ce ch aacacpaneen $ 270,000 $ 405,000...
-
Rob and Laura wish to buy a new home. The price is $300,000 and they plan to put 25% down. New Rochelle Savings and Loan will lend them the remainder at 8% per annum, compounded semi-annually for a...
-
Analysis for Financial Management (12th Edition) Chapter 5, Problem 16P
-
The primary purpose of the reformer is to convert methane and water to carbon monoxide and hydrogen (Equation 13.1). The extent of this reaction is limited by chemical equilibrium. where Subscript...
-
The combustion of 1.00 mol liquid octane (C 8 H 18 ), a component of gasoline, in excess oxygen is exothermic, producing 5.46 10 3 kJ of heat. (a) Write the thermochemical equation for this...
-
The thermite reaction produces a large quantity of heat, enough to melt the iron metal that is a product of the reaction: What is the enthalpy change if 50.0 g Al reacts with excess iron(III) oxide?...
-
Recent technological advances have led to the development of three new milling machines: brand A, brand B, and brand C. Due to the extensive retooling and startup costs, once a company converts its...
-
Gary Tuttle has Citiwide Insurance with 100% coverage after a $25.00 copay on office visits. His services today include an office visit ($62.00), urinalysis with differential ($65.00) and a Treadmill...
-
The Elgin Golf Dutton Golf Merger Elgin Golf Inc. has been in merger talks with Dutton Golf Company for the past six months. After several rounds of negotiations, the offer under discussion is a...
-
f ( x ) = x ^ 3 - 3 x ^ 2 - 2 4 x + 5 6 find all critical numbers
-
Suppose a beam of electrons is aimed at two slits in a slide placed in front of a screen. After a short time, the screen looks like the one at the right. a. What evidence does the picture give that...
-
On January 1, Mitzu Company pays a lump-sum amount of $2,700,000 for land, Building 1, Building 2, and Land Improvements 1. Building 1 has no value and will be demolished. Building 2 will be an...
-
Specify a template dependency for join dependencies.
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
A more accurate expression for E osc would be obtained by including additional terms in the TaylorMacLaurin series. The TaylorMacLaurin series expansion of f (x) in the vicinity of x 0 is given by...
-
The observed lines in the emission spectrum of atomic hydrogen are given by In the notation favored by spectroscopists, = 1/ = E/hc and R H =109,677 cm 1 . The Lyman, Balmer, and Paschen series...
-
Calculate the speed that a gas-phase fluorine molecule would have if it had the same energy as an infrared photon ( = 1.00 10 4 nm), a visible photon ( = 500. nm), an ultraviolet photon ( = 100....
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...
-
Pottery Ranch Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production at...

Study smarter with the SolutionInn App


