What is the sign of w for the following processes if they occur at constant pressure? Consider
Question:
What is the sign of w for the following processes if they occur at constant pressure? Consider only PV work from gases and assume that all gases behave ideally.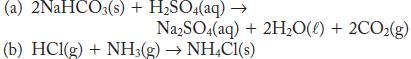
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
a w is ...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
What is the sign of w for the following processes if they occur at constant pressure? Consider only PV work from gases, and assume that all gases behave ideally.
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
Recession is defined as... a) increase in unemployment b) decrease in consumer spending c) two consecutive quarters of negative economic growth d) both A & B
-
For a given a hydrogen atom to be acidic, the C?H bond must be parallel to the p orbital?s of the C=O bond (that is, perpendicular to the plane of the adjacent carbonyl group). Identify the most...
-
Define the two major categories of quality cost and how they relate to each other.
-
Do we have excellent facilities for our R&D function?
-
Based on the information below, record the adjusting journal entries that must be made for Sufen Consulting on June 30, 2019. The company has a June 30 fiscal year-end. Use 18 as the page number for...
-
Import the text file PB Participants.txt as a table, using Tab delimeters and the General column format, into cell A3 in the current worksheet. Accept all other defaults.
-
Calculate w for the following reactions that occur at 298 K and 1 atm pressure. Consider only PV work from the change in volume of gas, and assume that the gases are ideal and the chemical equation...
-
When NaCl dissolves in water, can you accurately predict the sign of H for the dissolution of the soluble salt? Why or why not?
-
Comparing batting averages Three landmarks of baseball achievement are Ty Cobbs batting average of 0.420 in 1911, Ted Williamss 0.406 in 1941, and George Bretts 0.390 in 1980. These batting averages...
-
16. List I describes four systems, each with two particles A and B in relative motion as shown in figures. List II gives possible magnitude of their relative velocities (in m s) at time t = 3 S....
-
17. List I describes thermodynamic processes in four different systems. List II gives the magnitudes (either exactly or as a close approximation) of possible changes in the internal energy of the...
-
1. 2 mol of Hg(g) is combusted in a fixed volume bomb calorimeter with excess of O2 at 298 K and 1 atm into HgO(s). During the reaction, temperature increases from 298.0 K to 312.8 K. If heat...
-
3. A solution is prepared by mixing 0.01 mol each of H2CO3, NaHCO3, Na2CO3, and NaOH in 100 mL of water. pH of the resulting solution is [Given: pk, and pKa2 of H2CO3 are 6.37 and 10.32,...
-
6. Consider the following reaction. LOH red phosphorous Br2 R (major product) Br On estimation of bromine in 1.00 g of R using Carius method, the amount of AgBr formed (in g) is [Given: Atomic mass...
-
Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has: (a) Mg, (b) Ge, (c) Br, (d) V, (e) Y, (f) Lu.
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
Does the sun or the moon have the greater influence in causing tides?
-
A European driving from Paris to Brussels finds she has covered 291 km. How many miles is this?
-
For the circuit in Fig. 5.93 , find v o . 25 k2 100 k2 40 k2 20 k2 20 k2 10 k2 Vo +, 2 V 0+
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


