Question: The table below presents equilibrium distribution data for four gaseous solutes dissolved in water, using air as the carrier gas: a. Using a spreadsheet to
The table below presents equilibrium distribution data for four gaseous solutes dissolved in water, using air as the carrier gas:
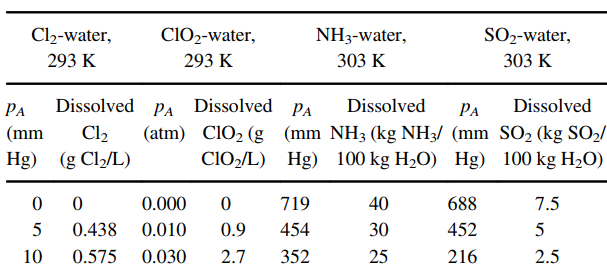
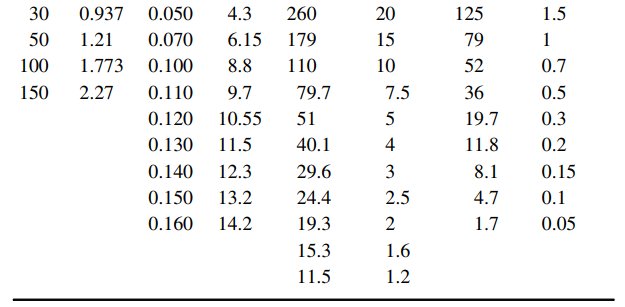
a. Using a spreadsheet to perform the calculations, prepare a graph of the equilibrium distribution data for each solute as partial pressure in the gas vs. molar concentration dissolved in the liquid (pA €“ cAL), and also in mole fraction coordinates (yA €“ xA) at 1.0 atm total system pressure. Which solute is the most soluble in water? Which solute dissolved in water can be stripped into air the easiest?
b. For each solute at the appropriate concentration range, estimate the Henry€™s law constant (H) based on the definition pA = H c*AL, and the distribution coefficient m based on the definition yA, = mx*A at 1.0 atm total system pressure.
Cl2-water, ClO2-water, NH3-water, SO2-water, 293 K 293 K 303 K 303 K Dissolved pA Dissolved PA Dissolved Dissolved PA PA (atm) ClO2 (g (mm NH3 (kg NH3/ (mm SO2 (kg SO2/ ClO/L) Hg) 100 kg H2O) Hg) 100 kg H2O) (mm Cl2 Hg) (g Cl/L) 0.000 40 688 719 7.5 0.438 30 452 0.010 0.9 454 5 10 0.575 2.7 352 25 216 0.030 2.5 30 0.937 0.050 4.3 260 20 125 1.5 50 1.21 0.070 6.15 179 15 79 100 1.773 0.100 8.8 110 10 52 0.7 150 2.27 0.110 9.7 79.7 7.5 36 0.5 0.120 10.55 51 19.7 0.3 0.130 11.5 40.1 4 11.8 0.2 0.140 12.3 29.6 3 8.1 0.15 0.150 13.2 24.4 2.5 4.7 0.1 0.160 14.2 19.3 1.7 0.05 15.3 1.6 11.5 1.2
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
a Equilibrium distribution curves ClO 2 Water T 29... View full answer

Get step-by-step solutions from verified subject matter experts


